Alexander S. Henderson, John F. Bower and M. Carmen Galan
Org. Biomol. Chem., 2020,18, 3012-3016
DOI:
10.1039/D0OB00155D,
Communication
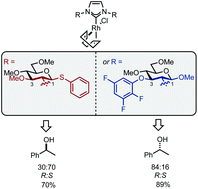
The practical synthesis of carbohydrate-based NHC–Rh complexes bearing C1 or C3 sterically differentiated positions, accessed by glycosylation or SNAr strategies, is reported. These catalysts exhibit pseudo-enantiomeric behaviour in the hydrosilylation of acetophenone. We show that steric bulk at C1 gives preference for (S)-phenyl-1-ethanol, while bulk at C3 leads to the (R)-enantiomer. These results represent the first example of pseudo-enantiomeric carbohydrate-based NHC ligands leading to enantiotopic discrimination.