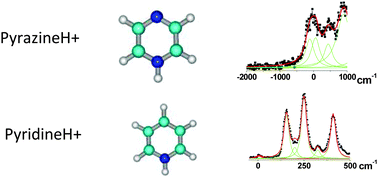
The excited state lifetimes of DNA bases are often very short due to very efficient non-radiative processes assigned to the ππ*–nπ* coupling. A set of protonated aromatic diazine molecules (pyridazine, pyrimidine and pyrazine C4H5N2+) and protonated pyrimidine DNA bases (cytosine, uracil and thymine), as well as the protonated pyridine (C5H6N+), have been investigated. For all these molecules except one tautomer of protonated uracil (enol–keto), electronic spectroscopy exhibits vibrational line broadening. Excited state geometry optimization at the CC2 level has been conducted to find out whether the excited state lifetimes measured from line broadening can be correlated to the calculated ordering of the ππ* and nπ* states and the ππ*–nπ* energy gap. The short lifetimes, observed when one nitrogen atom of the ring is not protonated, can be rationalized by relaxation of the ππ* state to the nπ* state or directly to the electronic ground state through ring puckering.