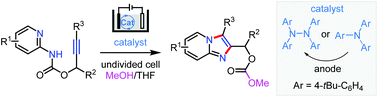
The development of electrocatalytic synthetic methods hinges on efficient molecular catalysts. Triarylamines are well-known redox catalysts because of the good stability of their corresponding amine radical cations. Herein we show that tris(4-(tert-butyl)phenyl)amine decomposes unexpectedly during electrolysis in MeOH/THF to afford a tetraarylhydrazine, 1,1,2,2-tetrakis(4-(tert-butyl)phenyl)hydrazine. In addition, we have applied this tetraarylhydrazine, which is either preprepared or formed in situ from tris(4-(tert-butyl)phenyl)amine, as an electrocatalyst for the synthesis of imidazopyridines and related N-heteroaromatic compounds through intramolecular [3 + 2] annulation. This metal-free electrocatalytic method provides straightforward access to the N-heteroaromatic compounds from readily available materials without the need for external chemical oxidants.