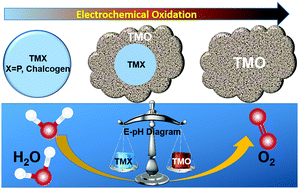
The oxygen evolution reaction represents an important electrochemical reaction in several energy storage and conversion devices such as water electrolyzers and metal–air batteries. Developing efficient, inexpensive and durable electrocatalysts for the oxygen evolution reaction (OER) has been one of the major focuses of applied electrochemistry and has attracted considerable research attention in the past decades. Non-oxide based transition metal compounds, typically transition metal phosphides (TMPs) and chalcogenides (TMCs), have recently emerged as new categories of OER pre-catalysts, demonstrated outstanding electrocatalytic performance as compared to the conventional oxide- or hydroxide-based OER catalysts for alkaline water electrolysis, and even shown promise to replace noble metals for proton-exchange membrane (PEM) water electrolysis. In this feature article, we will summarize the latest advances in the development of TMP- and TMC-based OER electrocatalysts. In particular, we will discuss the electrochemical stability of TMPs and TMCs predicted using Pourbaix diagrams and their morphological, structural and compositional evolution under OER conditions. We will also point out some challenges to be addressed in this specific area of research and propose further investigations yet to be done.