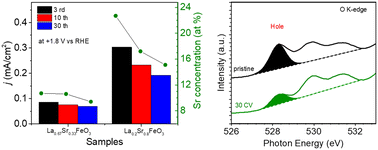
In this work, we have investigated the relationships between surface stability, electronic structure and O2 evolution reaction (OER) activity for epitaxial thin film La1−xSrxFeO3 (x = 0, 0.33, 0.8) model electrocatalysts before and after different electrochemical treatments. Cyclic voltammetry (CV) between +1.22 V and +1.92 V vs. RHE results in the continuous enhancement of OER performance of LaFeO3, while for La0.67Sr0.33FeO3 and La0.2Sr0.8FeO3 a gradual decrease of OER performance with increasing number of CV cycles was observed. A combination of atomic force microscopy, X-ray diffraction and X-ray reflectivity reveals that the surfaces of La1−xSrxFeO3 (x = 0, 0.33, 0.8) undergo surface morphology changes during OER treatment. Synchrotron ex situ X-ray photoemission spectroscopy data show a gradual down-shift of the Fermi level (EF) of LaFeO3 with increasing number of CV cycles, while near edge X-ray absorption fine structure spectroscopy (NEXAFS) at the Fe L-edge and O K-edge shows the presence of surface Fe4+ species as well as new hole states near the conduction band minimum upon electrochemical treatment, leading to a further enhancement of the electrochemical activity of LaFeO3. The newly formed hole state in LaFeO3 that appeared after 3 CV cycles remained constant upon progressing OER treatment. On the contrary, the decrease of OER performance of La0.67Sr0.33FeO3 and La0.2Sr0.8FeO3 with increasing CV cycles is attributed to an up-shift of EF along with a decrease of Fe4+ and hole state content after OER treatment. Furthermore, we found that the stability of the OER performance of La1−xSrxFeO3 is closely related to the leaching of Sr during OER, and the stability deteriorates with increasing Sr doping concentration in the pristine samples.