Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Driving diverse bond functionalisation with N-heterocyclic silylene-coinage metal–aryl complexes†
Moushakhi Ghosh a,
Kumar Gaurav‡
a,
Kumar Gaurav‡
 a,
Prakash Panwaria‡
a,
Prakash Panwaria‡ a,
Rishukumar Panday
a,
Rishukumar Panday a,
Srinu Tothadi
a,
Srinu Tothadi b and
Shabana Khan
b and
Shabana Khan *a
*a
aDepartment of Chemistry, Indian Institute of Science Education and Research Pune, Dr Homi Bhabha Road, Pashan, Pune-411008, India. E-mail: shabana@iiserpune.ac.in
bAnalytical and Environmental Sciences Division and Centralized Instrumentation Facility, CSIR-Central Salt and Marine Chemicals Research Institute, Gijubhai Badheka Marg, Bhavnagar-364002, India
First published on 30th June 2025
Abstract
Anchoring homolytic and heterolytic bond functionalisation at low-coordinate coinage metal centres is important due to their potential use as active catalysts in organic transformations. In the realm of carbene-stabilised coinage metal chemistry, heteroatom functionalised coinage metal precursors synthesised from such bond activations have long been explored. Interestingly, N-heterocyclic silylene, being an equally potent neutral donor ligand, has not been used for the same. Of note, carbene-stabilised heteroatom functionalised coinage metal precursors are vastly developed with copper centres only, while silver has been underexplored. This work reports the isolation of a variety of [PhC{N(tBu)}2SiN(SiMe3)2] (1) coordinated aryl-copper(I) and aryl-silver(I) complexes (2–8). We have also examined the reactivity pattern of organo-copper with differently substituted silylenes (9–11). These complexes were then utilised to cleave various homolytic and heterolytic bonds to access silylene-coordinated heteroatom functionalised coinage metal complexes (12–24). We have shown the reaction of reactive aryl-coinage metal precursors towards homolytic bonds, having B–B and Se–Se bonds, which led to the formation of an NHSi-supported dimeric μ-boryl bound Cu(I) complex (12) and a new class of unprecedented NHSi-supported coinage metal-selenogenolates (14–16). These aryl-coinage metal precursors also smoothly afforded several elusive NHSi-copper and silver amides (17–22) via N–H bond cleavage. A heterolytic cleavage of the P–Si bond resulted in the formation of NHSi stabilised copper and silver phosphide complexes (23 and 24), among which the latter is the first precedent of the dimeric Ag-phosphide complex. Lastly, we have utilised NHSi → copper–aryl complexes as aryl transfer reagents in C–C coupling reactions, which led to the formation of products in excellent yields with a high TON. The analogous silver complex was employed in the three-component α-aminonitrile synthesis efficiently. Our report establishes NHSi coordinated aryl copper and silver complexes as a perfect and robust platform for accessing a diverse array of reactive coinage metal precursors that were hitherto unknown.
1 Introduction
The N-heterocyclic silylenes (NHSis), known as heavier congeners of N-heterocyclic carbenes (NHCs), are now considered one of the formidable classes of neutral donor ligand systems.1 Over the years, several NHSis have been isolated and utilised in stabilising reactive main group species,2 coordinating transition metals for homogeneous catalysis,3 and activating small molecules.1c,4 Groundbreaking studies by various pioneering scientists have empowered NHSis as one of the promising ligand motifs for stabilising numerous elusive and reactive species.1e,5 Despite their superior catalytic activities in several organic transformations, the NHSi stabilised coinage metal complexes are comparatively less studied than the NHC ones.6 However, recent years have witnessed a growth in isolating differently functionalised NHSi-coordinated coinage metal complexes and exploring their catalytic activities (Chart 1a).7 Given our particular interest in this field,7a–g we continue to be intrigued by the future possibilities of NHSi-based coinage metal complexes as useful synthons beyond the coordination of coinage metal halides to encompass a broader range of synthetic possibilities. The coordination of NHSis with coinage metals having heteroatom functionalisation remains uncharted territory until now. Many NHC-based copper [Cu(I)] synthons have been isolated and studied for bond activation and homogeneous catalysis.8 Interestingly, the analogous situation with silver [Ag(I)] is far more underexplored. This is primarily because of the higher thermal decomposition of Ag(I) complexes under ambient conditions. Also, carbene–Ag(I) complexes are chosen precursors for transmetallation reactions with other transition metals, such as Cu, Au, Fe, Ni, and Co.9 The bond strength between carbenes and transition metals is greater than that of carbene–Ag(I) complexes, facilitating a smoother transmetallation process. In this context, aryl–coinage metal precursors(I) are a very reactive class of complexes,8c,10 which can be explored in bond activations due to the lability of E–aryl bonds (E = Cu and Ag). Also, NHC-based Cu(I) complexes with anionic C-donor atoms have been explored for their catalytic application and functionalisation reactions, such as the formation of Cu–X bonds (X = –SR, –PR2, –OR, and –NR2), intramolecular hydroalkoxylation of alkynes, the CuAAC reaction, and defluoro-borylation of fluoroalkenes.8p However, such utilisation of carbene–Ag(I)–aryl complexes is missing in the literature. Very recently, the NHC-coordinated Ag(I)–mesityl complex has been used as a precursor for crafting graphitic carbon nitride (g-C3N4),12 paving the way for their further exploration in organometallic and material chemistry. All these findings inspired us to isolate the NHSi-based Cu(I) and Ag(I)–aryl complexes (2–8), creating a platform for the further functionalisation of the labile Cu/Ag–aryl bonds (Chart 1b). In this work, we have demonstrated the coordination behaviour of aryl–coinage metal precursors exclusively with benzamidinato silylene, [PhC{N(tBu)}2SiN(SiMe3)2] (1), and then showcased the reactivities of these precursors towards various homolytic and heterolytic bond cleavage reactions (12–24) (Chart 1b). We have also briefly checked the reactivity pattern of different NHSis (9–11) with organocopper complexes to establish the breadth of the methodology. While the reactions of NHSi with Cu(I)–aryl precursors afforded di-coordinated NHSi → Cu–aryl complexes (2–5) smoothly, the reaction of Ag(I)–mesityl afforded a unique Ag2C ring with argentophilic interaction (6). The stability of the complex is interesting because of the high level of angle strains and torsional strains of the thermodynamically unstable three-membered Ag2C ring. Interestingly, there is no precedent of such a complex with NHSis to date. However, the reactions with other Ag(I)–aryl precursors yielded nearly linear coordinated complexes 7 and 8 due to the enhanced steric effects of aryl groups. The isolation of these complexes created a perfect platform for bond functionalisation. Inspired by Sadighi's isolation of NHC-supported Cu(I)–boryl complexes [(IPr)Cu(Bpin), (ICy)Cu(Bpin)], catalytically active for CO2 reduction and a few isolated carbene-stabilised copper–boryl complexes,13 we isolated NHSi–Cu–boryl complexes via diboron bond cleavage, which yielded complex 12 with a dimeric μ-boryl framework. Additionally, given the scarcity of NHC/CAAC-supported chalcogenides14 and the absence of NHSi-coordinated coinage metal chalcogenolates, we also reacted NHSi → Cu(I)/Ag(I)–aryl complexes with different diselenides, leading to the isolation of NHSi–Cu/Ag selenogenolate (14–16). Very recently, we have shown that the NHSi → Cu–amides can demonstrate fascinating luminescence properties.15 Along with this, N–H bond cleavage is also an important part of cross-coupling reactions; hence, we attempted syntheses of Cu/Ag–amides (17–22).16 Lastly, employing the σ-bond metathesis reaction of Cu/Ag–C and P–Si bonds, we were able to isolate rarely occurring Cu(I) and Ag(I) phosphide complexes (23–24). Meanwhile, NHC–Cu–phosphides have been reported only in limited instances.8i To the best of our knowledge, the precedence of low-coordinate Ag–phosphide is still elusive. Overall, this vast piece of work intends to highlight the synthetic versatility and facile bond activation potential of NHSi–Cu/Ag–aryl complexes towards various energy demanding bonds such as B–B (∼462.86 kJ mol−1), Se–Se (∼239.48 kJ mol−1), N–H (∼387.41 kJ mol−1), and P–Si (∼263.58 kJ mol−1) (Table S4, see the ESI for the details†). To understand the bonding nature of all the newly synthesised complexes, density functional theory (DFT) calculations were performed using the Gaussian 09 program package (see the ESI for details†), and the description of theoretical input is provided at the relevant places. | ||
| Chart 1 (a) Reported benzamidinato silylene coordinated Cu(I) complexes to date. No NHSi-coordinated Cu(I)/Ag(I)–aryl complex is reported;7e,g,i,j,11 (b) overview of the present work. | ||
To establish the significance of such organo-coinage metal precursors in catalytic reactions, we performed a few brief proof-of-concept catalytic model reactions, such as C–C cross-coupling reactions and a three-component α-aminonitrile synthesis, which afforded the desired compounds in excellent yields (vide infra).
2 Results and discussion
2.1 Synthesis of NHSi–E–aryl (E = Cu and Ag) complexes
The 29Si{1H} spectra display a broad peak at δ 6.5 and δ 6.6 ppm for the Si(II) → Cu centre for complexes 2 and 3–5, respectively. The molecular structures of 2–5 demonstrate that the Si(II) centres adopt a distorted tetrahedral geometry (Fig. 1). The Si–Cu bond lengths are 2.2590(8) Å (complex 2), 2.2407(8) Å (complex 3), 2.246(2) Å (complex 4), and 2.242(1) Å (complex 5), which are shorter than the previously reported Si–Cu bond length in the carbazole-substituted benzamidinato silylene stabilised dimeric Cu2Br2 complex (2.203(2) and 2.212(2) Å),7c and longer than that in [PhC{N(tBu)}2SiN(SiMe3)2] stabilised Cu2Br2 (2.222(2) Å). But they match closely with that in the dimeric Cu2I2 complex of [PhC{N(tBu)}2SiN(SiMe3)2] (2.243(3) and 2.250(3) Å).6a They are significantly longer than the predicted bond length for the NHSi → Cu(I) complex (2.061 Å) by Frenking and co-workers.18 It was observed that the Si–Cu bond lengths decrease with increasing steric bulk around the aryl group, following the trend 2 > 4 > 5 > 3. They feature almost linear geometry around the Cu(I) centre featuring a bond angle Si1–Cu1–C1 of 173.28(6)° (complex 2), 170.72 (7)° (complex 3), 172.7(1)° (complex 4) and 172.5(1)° (complex 5) which is in accordance with the previously reported IMes (1,3-bis-(2,4,6-trimethylphenyl)imidazole-2-ylidene) stabilised Cu–mesityl complex [173.53(9)°]19 but the Si–Cu–C angles of complexes 2–5, are shorter than that of the carbene coordinated copper-mesityl complexes (in the range of [174.53(6)–178.06(12)°]).19,20 To understand the implications of these bonding patterns on the electronic structure, the frontier molecular orbital (FMO) analysis was carried out (Fig. S111†). This reveals that the highest occupied molecular orbital (HOMO) is primarily localised over the Cu d orbital and the π orbital of the mesityl group. Conversely, the electron density in HOMO−1 predominantly resides over the Cu(I)–Cipso bond, with a marginal distribution extending over the Si(II) → Cu bond and the mesityl group. Notably, HOMO−3 is majorly localised over the Si(II) → Cu bond, exhibiting a minor dispersion over the Cu(I)–Cipso bond and the amidinate fragment. The HOMOs of complexes 3 and 4 (see the ESI for computational details†) show a predominant contribution from the Cu(I)–Cipso bond and a minor involvement from the Si(II) → Cu bond and the aryl group. It is important to note that the electron density on Cipso is more in complex 3 (35%) and 4 (35%) in the HOMO than in complex 2 (27%), indicating the stronger electron-donating effect of Dipp (2,6-diisopropylphenyl) and Trip(2,4,6-triisopropylphenyl) over the Mes (2,4,6-trimethylphenyl) group. However, the lowest unoccupied molecular orbital (LUMO) for all complexes is predominantly localised over the benzamidinato fragment.
 | ||
| Fig. 1 Molecular structures of 2 (a), 3 (b), 4 (c), and 5 (d). The bond distance (Å) and bond angle (°) are discussed in the ESI.† | ||
 | ||
| Fig. 2 Molecular structures of 6 (a), 7 (b) and 8 (c). The bond distance (Å) and bond angle (°) are discussed in the ESI.† | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio, which furnished complexes 9, 10, and 11, respectively.29 We selected these variations based on their differences in electronic and steric parameters, anticipating diverse reactivities. The formation of 9 proceeded through σ-bond metathesis at the labile Si–Cl moiety, and the mesityl group migrated to the Si(II) centre to form a dimeric Cu2Cl2 core (Fig. S136†). The peak at δ 32.7 ppm in the 29Si{1H} NMR spectrum is more deshielded than the previously reported dimeric copper(I) chloride of compound 1 (δ 4.79 ppm),11f which might be due to the presence of the σ-donating mesityl group at the silicon(II) centre. 9 crystallises in the monoclinic P21/n space group (see Fig. S136†) and is isostructural to the dimeric Cu2Cl2 complex of 1.11f
1 molar ratio, which furnished complexes 9, 10, and 11, respectively.29 We selected these variations based on their differences in electronic and steric parameters, anticipating diverse reactivities. The formation of 9 proceeded through σ-bond metathesis at the labile Si–Cl moiety, and the mesityl group migrated to the Si(II) centre to form a dimeric Cu2Cl2 core (Fig. S136†). The peak at δ 32.7 ppm in the 29Si{1H} NMR spectrum is more deshielded than the previously reported dimeric copper(I) chloride of compound 1 (δ 4.79 ppm),11f which might be due to the presence of the σ-donating mesityl group at the silicon(II) centre. 9 crystallises in the monoclinic P21/n space group (see Fig. S136†) and is isostructural to the dimeric Cu2Cl2 complex of 1.11f
The silicon(II) centre of 9 attains a distorted tetrahedral geometry with a Si–Cu bond length of 2.213(1) Å.11f On the other hand, [PhC{N(tBu)}2SiOtBu] furnishes μ-dimeric mesityl-bridged Cu(I) complex, 10 (Scheme 2). The 29Si{1H} NMR spectrum shows a broad peak at δ 3.8 ppm, deshielded from [PhC{N(tBu)}2SiOtBu] (δ−5.0 ppm),28b but comparable to the dimeric Cu2Cl2 complex of 1 as mentioned above. Complex 10 crystallises in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group with a Si–Cu bond length of 2.249(1) Å, and the Cu–C bond lengths are 2.170(4) and 2.132(4) Å, suggesting the μ-bridging fashion (Fig. 3). The tri-coordinated Cu(I) adopts a Si1–Cu1–C1 bond angle of 119.8(1)°. The Cu⋯Cu distance in 10 is 2.335(6) Å, which is shorter than the Cu⋯Cu distance (2.46 Å) in mesityl copper.10 To broaden the scope of the reactivity pattern, we performed a reaction of mesityl copper with newly synthesised [PhC{N(tBu)}2Si{N(Dipp)SiMe3}] and obtained complex 11, similar to complex 2. Formation of complex 11 was confirmed by 29Si{1H} NMR, which shows a broad peak at δ 11.8 ppm, which is further deshielded than [PhC{N(tBu)}2Si{N(Dipp)SiMe3}] (δ 2.98 ppm). The Si–Cu distance in complex 11 is observed to be 2.227(2) Å, shorter than that in complex 2 {2.2590(8) Å}, and the Cu–C distance is 1.934(3) Å, with a bond angle of 179.68(9)° around Si–Cu–C centres (Fig. 3). The abovementioned reactivity of different silylenes clearly outlines the influence of steric and electronic properties on the structure of the final product.
space group with a Si–Cu bond length of 2.249(1) Å, and the Cu–C bond lengths are 2.170(4) and 2.132(4) Å, suggesting the μ-bridging fashion (Fig. 3). The tri-coordinated Cu(I) adopts a Si1–Cu1–C1 bond angle of 119.8(1)°. The Cu⋯Cu distance in 10 is 2.335(6) Å, which is shorter than the Cu⋯Cu distance (2.46 Å) in mesityl copper.10 To broaden the scope of the reactivity pattern, we performed a reaction of mesityl copper with newly synthesised [PhC{N(tBu)}2Si{N(Dipp)SiMe3}] and obtained complex 11, similar to complex 2. Formation of complex 11 was confirmed by 29Si{1H} NMR, which shows a broad peak at δ 11.8 ppm, which is further deshielded than [PhC{N(tBu)}2Si{N(Dipp)SiMe3}] (δ 2.98 ppm). The Si–Cu distance in complex 11 is observed to be 2.227(2) Å, shorter than that in complex 2 {2.2590(8) Å}, and the Cu–C distance is 1.934(3) Å, with a bond angle of 179.68(9)° around Si–Cu–C centres (Fig. 3). The abovementioned reactivity of different silylenes clearly outlines the influence of steric and electronic properties on the structure of the final product.
 | ||
| Fig. 3 Molecular structures of 10 (a) and 11 (b). The bond distance (Å) and bond angle (°) are discussed in the ESI.† | ||
2.2 Reactivities of NHSi-based E–aryl complexes (2 and 7) towards various bond cleavage reactions
Based on various structural motifs obtained (2–11) through the coordination ability of differently functionalised silylene with organo-coinage metal precursors, complexes 2 and 7 were chosen for further exploration in a range of bond activation reactions.Thus, we performed a reaction of less sterically bulky bis(catecholato)diboron (B2cat2) with complex 2 (Scheme 3) in toluene. In this case, we could obtain dimeric [PhC{N(tBu)}2SiN(SiMe3)2}2Cu2B2Cat2] (12) and Mes–Bcat (13) as a mixture of the products. The isolation of complex 12 also indicated the higher reactivity of sterically less hindered B2cat2, which was previously pointed out.34 The molecular structure of complex 12 (Fig. 4) established the μ-coordination mode of the boryl linkage to Cu(I) coordinated with NHSi unambiguously. However, the poor quality of the crystals prevented us from discussing the metrical parameters. Analogous NHC stabilised μ-boryl Cu(I) complexes were reported by Sadhigi, Kleeberg, and co-workers, which show a μ-boryl Cu(I) core (Chart 2).35 We could not get a clean 1H NMR spectrum of 12 as it was isolated as a mixture with 13 (see the ESI†). However, 29Si{1H} NMR spectra revealed two sharp singlets at δ 6.3 and 7.1 ppm, corresponding to the –SiMe3 moiety, and a singlet at δ 3.7 ppm, presumably indicating the Si(II)–Cu(I) bond. Although there are a few Cu–μ-boryl complexes reported in the literature, the understanding of the associated FMOs is largely missing, apart from the work by Tilley and co-workers.35e Drawing inspiration from this, we analysed its electronic structure, which reveals that the HOMO of the dimeric Cu(I)–μ2–Boryl complex (12) (Fig. S114†) is localised over the Cu2B2 ring. The orbital composition of the HOMO shows that the localisation of electron densities at Cu centres is 19 and 13%, while at B centres, it is 30 and 22%, respectively. This 3c–2e bonding situation was further confirmed by the natural localised molecular orbital (NLMO) analysis, showing that the contribution of each Cu centre and B orbital in the Cu–B–Cu bond is 13, 6, and 73%, respectively. HOMO−1 also has a contribution over the Cu2B2 ring along with the Si(II) → Cu bond, whereas the electron density in the HOMO−2 is predominantly localised over the Si(II) → Cu bond, along with the contribution from the catechol–boryl moiety.
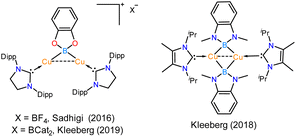 | ||
| Chart 2 Previously reported examples of NHC-supported μ-boryl Cu(I) complexes.34,35 | ||
The reaction pathway shows that complex 2 forms an intermediate (Int) involving non-covalent interaction between boron atoms of B2pin2 and the mesityl ring (Fig. 5), proceeding via an intermediate to give complex 12 (detailed analyses provided in the ESI†). Interestingly, the preparation of aryl boronate esters either involves Suzuki–Miyaura cross-coupling for C–B bond formation36 or base-mediated ArB(OH)2 formation.37 Thus, the formation of 13 as a byproduct of this reaction in the crystalline form gives easy access to mesityl boronic ester (see the ESI†). It is important to note that a similar reaction with complex 7 failed to yield the NHSi → Ag–boryl complex, likely due to poor thermal stability and rapid dissociation of the Ag–B bond. Although we attempted to monitor the fate of the product using 29Si{1H} NMR spectroscopy, we were able to detect a Si(II)–bound Ag(I) species. However, the exact nature of the species formed alongside Trip–Bcat remains unclear at this stage.
 | ||
| Fig. 5 Relative energy (kcal mol−1) (not drawn to scale) reaction profile for the formation of complex 12 from complex 2 with B2cat2. | ||
We also tried the reaction of complex 2 with other diboron agents discussed in the ESI.†
 | ||
| Fig. 6 Molecular structures of 14 (a), 15 (b), and 16 (c). The bond distance (Å) and bond angle (°) are discussed in the ESI.† | ||
It was found that 14 is stable in the dimeric form up to the temperatures of 380 and 420 K in the solution and gas phase, respectively (see the ESI for computational details†). The Int observed in the reaction pathway for the formation of complex 14 (Fig. 7) is stabilized by the interaction between Cu and Se centers, C–H⋯Se, C–H⋯π, and tetrel bonding. These non-covalent interactions favour the formation of the Cu–Se bond and the breaking of the Cu–Cipso bond, which is visualised in the TS, and confirmed by the relevant geometrical parameters and NBO analysis (see the ESI for details†).
 | ||
| Scheme 5 Syntheses of various NHSi-coordinated metal amide complexes [monodentate amides (a), bidentate amides (b), and perfluorinated amides (c)] from 2 and 7. | ||
Complex 17, formed by reacting 2 with pyrrole, shows a broad peak at δ 7.8 ppm in the 29Si{1H} NMR spectrum, and its structure was confirmed by single-crystal X-ray diffraction. The Si1–Cu1 bond length for complex 17 was found to be 2.231(3)Å, and the Cu–N bond length was 1.878(7) Å. Complex 17 features an almost linear Si–Cu–N bond angle of 172.2(5)° (Fig. 8). Similarly, reacting 2 with 2-mesityl-1H-pyrrole yielded complex 18, displaying a29Si{1H} peak at δ 7.4 ppm, deshielded relative to 2 due to the π-electron-donating mesityl group. Complex 18 has a Si1–Cu1 bond length of 2.203(1) Å and a Cu1–N1 bond length of 1.874(3) Å (Fig. 8), with a nearly linear Si1–Cu1–N1 angle of 177.5(1)°. The Si–Cu bond distance is comparable to that of the heteroleptic [Cu(tmeda)(PhC(NtBu)2Si{OtBu})][OTf] (tmeda = N,N,N,N-tetramethylethylenediamine) complex [2.2003(6) Å]. Furthermore, we have performed FMO visualisation along with NBO analysis for all the isolated NHSi → E–amide complexes (17–22) to elucidate the nature of the bonding. The HOMO of complex 17 is located over the pyrrolato ring whereas HOMO−1 and HOMO−2 have a major contribution from the Si(II) → Cu(I) bond (Fig. S116†). For 18, the HOMO is localised on the 2-mesitylpyrrolide moiety, HOMO−1 on the Cu(I)–Namide fragment, and HOMO−2 across the Si(II) → Cu bond (Fig. S116†). After isolating complexes 17 and 18, we were curious to investigate the coordinative variability of N-donor functionalised secondary amines. To explore the coordination ability of N–H systems containing auxiliary N-donor atoms, we obtained a few coordinated Cu(I) complexes (19 and 20) (Scheme 5b). The three-coordinate NHSi–copper(I) complex (19) was isolated by treating 2 with N-mesitylpyridin-2-amine, which gives a broad peak at δ 6.9 ppm for the Si(II) centre in the 29Si{1H} NMR spectrum. Complex 19 features a Si1–Cu1 bond length of 2.1964(8) Å (Fig. 8), which is shorter than the Si(II) → Cu(I) bond in complex 18. The Cu1–N5 bond length of 19 is 2.654(2) Å, which is significantly longer than the reported IPr coordinated three copper(I) complexes attached to the 2-(2,3,4,5-tetrafluorophenyl) pyridine group (2.185(4) Å).45 Thompson and co-workers isolated a few (NHC)Cu(py2BMe2) {py2BMe2 = di(2-pyridyl)dimethylborate} complexes, which feature N^N donation to the Cu(I) centre. These complexes possess Cu–N bond lengths ranging from 1.9929(16) to 2.0288(15) Å.46 The Si1–Cu1–N1 bond angle in 19 is 172.44(7)°, which deviates from linearity due to the additional N-donation. Stalke and co-workers isolated N-mesitylpyridin-2-amine substituted benzamidinato silylene coordinated trimeric Cu(I) halide complexes,47 which also feature pyridyl N-donation to the Cu(I) centre with N → Cu bond lengths from 1.995(4) to 2.009(2) Å. These bonds are significantly shorter than the observed N → Cu bond in 19, denoting a greater extent of electron donation in the former one. The amidinate ligands are infinitely versatile in terms of potential structure and substitution patterns.48 This prompted us to isolate complex 20 containing the Dipp group substituted formamidine moiety (Scheme 5b) as an example of auxiliary N-donor coordinated secondary amines. This features a broad peak at δ 6.6 ppm for Si(II) attached to three-coordinate Cu(I) centres in the 29Si{1H} NMR spectrum. Due to an additional N-donor atom, the Si(II) centre is more shielded than the complexes 17 and 18. Complex 20 is structurally reminiscent of the CAAC stabilised three-coordinate formamidinate copper(I) complex (Fig. 8).49 The molecular structure of 20 reveals a Si1–Cu1–N1 angle of 171.24(9)°, whereas, for the CAAC one, the C–Cu–N angle is 175.09(7)°.49,50 The Si1–Cu1 and Cu1–N1 bond lengths are 2.194(1) and 1.903(3) Å, respectively. The Cu1–N2 bond length is 2.712(3) Å, shorter than the sum of their van der Waals radii (2.94 Å), suggesting the coordinative nature. This is also shorter than the formamidinate Cu(I) complex of CAAC (Cu–N 2.912(14) Å).49 The C–N bond lengths in the formamidinate backbone are different, with long N1–C13 and short N2–C13 distances of 1.337(4) and 1.294(4) Å, respectively. FMO visualisation of 19 and 20 shows that the HOMO of complex 19 shows that there is a major contribution from the 2-mesitylpyridyl moiety and d orbital of the Cu atom (Fig. S117†). The electron density at the HOMO−1 is predominantly localised over the Cu–Namide bond with a minor contribution over the pyridine ring, whereas the electron density is delocalised over the Si(II) → Cu–N–mesityl moiety in HOMO−2 with a minor contribution from the pyridine ring (Fig. S117†). However, for complex 20 the HOMO is majorly located over the formamidine moiety whereas HOMO−2 and HOMO−4 are spread over the Si(II) → Cu bond (Fig. S117†). NBO calculation further shows that the N → Cu bond is stabilised by E(2) values of 3.80 and 4.54 kcal mol−1 for 19 and 20, respectively (Fig. S122a and b†). While there could be possibilities of numerous secondary amide groups in the literature, N–H bond activation of bis-perfluoroamine is of particular interest because of its weak donation ability to the metal centre.51 Treatment of complex 2 with bis-pentafluorophenylamine in toluene yielded complex 21 (Scheme 5c), with a Si1–Cu1 bond length of 2.220(1) Å and a Si1–Cu1–N1 angle of 169.3(1)° (Fig. 8), deviating from linearity due to ortho-fluorine Cu⋯F contacts (Cu1–F10: 2.791(3) Å and Cu1–F5: 2.727(2) Å). These interactions elongate the corresponding C–F bonds [C–F5: 1.350(6) Å and C–F2: 1.358(3) Å]. Complex 21 shows the peak at δ 7.1 ppm for the Si(II) centre in the 29Si{1H} NMR spectrum. As a proof of concept, the reaction of complex 7 with bis-perfluoroarylamine produces a structurally identical complex of 22 (Scheme 5c). The Si–Ag bond length in complex 22 is 2.3543(8) Å, longer than the Si–Cu bond length in complex 21. The intermolecular π-stacking between the phenyl group of the amidinate moiety and the perfluoro-substituted benzene ring further stabilises complexes 21 and 22. Also, complex 21 features intermolecular F⋯F interaction (see the ESI†). The MOs of these complexes are reminiscent of complex 18 (Fig. S118†). However, the Cu⋯F interactions in complex 21/22 are supported by the E(2) values of 0.70/2.26 and 3.06/1.04 kcal mol−1 for F5 and F10, respectively (Fig. S122c and d†).
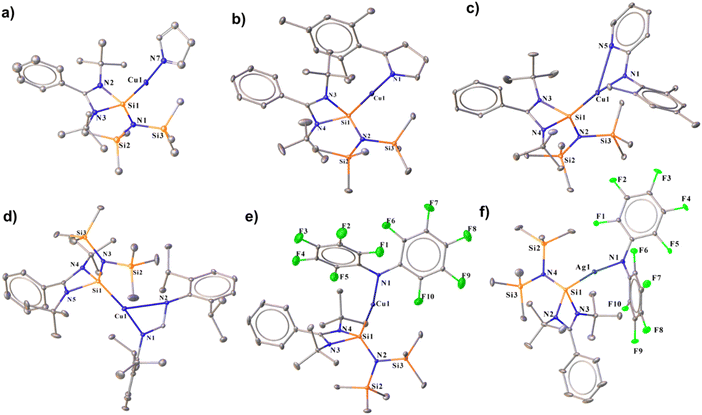 | ||
| Fig. 8 Molecular structures of complexes 17 (a), 18 (b), 19 (c), 20 (d), 21 (e), and 22 (f). The bond distance (Å) and bond angle (°) are discussed in the ESI.† | ||
An apparent trend emerges after observing the chemical shifts of 29Si{1H} NMR and bonding parameters of the Cu–amide complexes (17–21) with 2 (Table 1). We observe a deshielding effect on the Si(II) centres for complexes 17–21 that can be attributed to the electrophilic nature of the amide group. Furthermore, the Si(II) → Cu and Cu–Namide bond lengths of complexes 17–21 are shorter than the corresponding Si(II) → Cu and Cu–Cipso bond lengths in complex 2. This discrepancy suggests an enhanced electropositive character of the Cu(I) centre in complexes 17–21, as also realised from the Mulliken charge analysis (Table 2) on the Si, Cu, Cipso, and Namide. It was found that the electropositive character of the Cu(I) centre indeed increases in 17–21 with a gradual increase in the electrophilic nature of the amide ligand as compared to the mesityl group of 2. As a proof of concept, we investigated the reaction pathway of complex 17. Interestingly, the reaction profile (Fig. 9) for the formation of complex 17 shows the hydrogen transfer of the pyrrole group to the mesityl ipso carbon in the transition state (TS), which was confirmed by the imaginary frequency at 1373 cm−1 along these reaction coordinates. Furthermore, the covalent nature of the Cipso–H and N–H bonds is confirmed by the enormously strong C–H⋯N hydrogen bond in the TS with an E(2) energy of 134.62 kcal mol−1 (details in the ESI†).
| Complex | 29Si{1H} chemical shift (δ) ppm | Si(II) → Cu(I) bond length (Å) | Cu–C/N bond length (Å) |
|---|---|---|---|
| 2 | 6.5 | 2.2509(8) | 1.946(2) |
| 17 | 7.8 | 2.231(3) | 1.878(7) |
| 18 | 7.4 | 2.203(1) | 1.874(3) |
| 19 | 6.9 | 2.1964(8) | 1.906(2) |
| 20 | 6.6 | 2.194(1) | 1.903(3) |
| 21 | 7.1 | 2.220(1) | 1.929(4) |
| 2 | 17 | 18 | 19 | 20 | 21 | 22 | |
|---|---|---|---|---|---|---|---|
| qSi | 0.617 | 0.830 | 0.793 | 0.857 | 0.837 | 0.820 | 0.599 |
| qM | −0.474 | −0.342 | −0.193 | −0.214 | −0.203 | −0.227 | −0.060 |
| qC/N | −0.183 | −0.115 | −0.209 | −0.248 | −0.240 | −0.239 | −0.354 |
The molecular structures of both complexes reveal a dimeric μ-bridged Cu(I)/Ag(I)–PPh2 core coordinated with two NHSi moieties from each end (Fig. 10). The formation of 23 and 24 resulted from eliminating Mes–SiMe3 (for 23) and Trip–SiMe3 (for 24). However, these eliminations are not very facile under ambient conditions, hence dropping the yield of the desired product. The dimeric core represents a near rhomboid geometry with bond angles for 23 [P1–Cu1–P1 86.16° and Si1–Cu1–P1 136.98°] and 24 [P1–Ag1–P1 84.48° and Si1–Ag1–P1 137.49°]. The Si–Cu(I)/Ag(I) bond distances are 2.2758(8) and 2.401(1) Å for 23 and 24, respectively, which are longer than that in the NHC → Cu–PR2 complexes [1.9272(18) Å for the monomeric (6-Dipp)CuPPh2 complex and 1.9234(15) Å for the dimeric IDippCuPPh2 complex] reported in the literature.8i The Cu–P distance [2.3799(8) Å] in complex 23, is longer than that in the monomeric (6-Dipp)CuPPh2 complex [2.2113(5) Å] and is in line with that of the dimeric IDippCuPPh2 complex [2.3298(5) Å].8h,i,54 The Ag–P bond distance [2.537(1) Å] in complex 20 is in line with the Ag–P bond distances reported in [Ag12(PSiMe3)6(iPr2-bimy)6] and [Ag26P2(PSiMe3)10(iPr2-bimy)8] [2.579(3) and 2.605(5) Å].53 The intermetallic distances (E⋯E) are significantly longer [3.458 Å (complex 23) and 3.791 Å (complex 24)], suggesting the absence of metallophilic interactions in these complexes, unlike those in complex 14. Since such low-coordinate Cu(I)–phosphide complexes are limited only to their structure elucidation, it became customary for us to understand the electronic structures of complexes 23 and 24 using FMO visualisation. The HOMO and HOMO−1 of both complexes were composed of the Cu/Ag–P bonding interaction with d–p orbital overlap, whereas HOMO−4 resides over Si(II)–Cu/Ag(I) bonds (Fig. S119†). Isolation and characterisation of NHSi → coinage metal–phosphides are the first of their kind, thus indicating future possibilities of fine-tuning and utilisation in homogeneous catalysis. The reaction pathway shows that C–H⋯π and C–H⋯P hydrogen bonding stabilises the Int observed in the reaction profile (Fig. 11) for the formation of complex 23. The strengthening of the interaction between P and Cu centers in the TS suggests the bond formation between P⋯Cu centers. Apart from their brief mention here, a complete discussion of the computed pathways, including energetic profiles and structural details, is provided in the revised ESI (pages S102–S110†). We performed detailed DFT calculations on four representative complexes of each bond type among 12–24, as already mentioned. These complexes were selected to reflect the four distinct types of bond activations investigated: homolytic B–B (12) and Se–Se (14), and heterolytic N–H (17) and P–Si (23) bond activations. We believe the selected cases will provide insight into the general reactivity patterns. All the mechanistic pathways show a similar fashion of nucleophilic attack of the reactant to the Cu(I) centre in the transition states, followed by the mesityl group migration to the electrophilic substituent, affording the desired complexes.
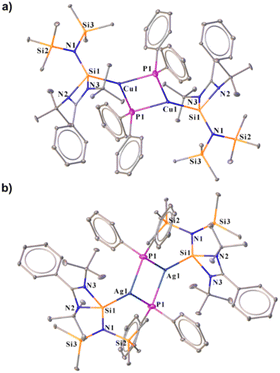 | ||
| Fig. 10 Molecular structures of complexes 23 (a) and 24 (b). The bond distance (Å) and bond angle (°) are discussed in the ESI.† | ||
2.3 The catalytic utility of NHSi-organo-coinage metal complexes towards different organic transformations
The examples of the NHSi ligated transition metal mediated catalysis are limited to a handful of catalytic reactions.55 Recently, our group has demonstrated the scope of NHSi ligated coinage metal complexes in a few different organic reactions, like azide–alkyne cycloaddition,6a,7e,47 glycosidation,7f and three-component coupling reactions.7d Motivated by our previous results on catalysis, we aimed to probe the catalytic performances of the isolated NHSi–organocoinage metal complexes. Our brief attempt at these complexes towards catalysing organic transformations is equally promising. | ||
| Chart 4 Previous reports on C–C coupling reactions with NHSi as a ligand on the metal centre. (cod = cyclooctadiene). | ||
Since we already had well-characterised NHSi–Cu–aryl systems (2 and 5), we utilised them as an efficient aryl group transfer agent for Pd-catalysed C–C coupling reactions with aryl iodides (Scheme 7).
 | ||
| Scheme 7 Pd-catalysed sp2–sp2 C–C coupling reaction using 2 and 5 as an aryl transfer reagent. *Isolated yields. | ||
The C–C coupling reactions with a few aryl iodides were carried out under the optimised conditions with complex 2. We observed good to excellent product yields for the heteroaromatic (IV and VIII) and fused aromatic (V and IX) systems (Scheme 7). Using the NHSi coordinated organo–copper complex as an alternative to conventional aryl boronic esters provides a lower Pd catalyst loading (0.5 mol%) compared to the conventional C–C coupling reactions.59 It is important to note that the Pd catalyst loading (5 mol%) for the C–C coupling reaction by Uchiyama and co-workers remains much higher with the use of additional ligand loading (up to 15 mol%) (Table 3).
| Serial no. | Pd-based catalyst (mol%) | Temperature (°C) | Yield (%) | TON |
|---|---|---|---|---|
| 1 | 5 | 80–140 | 91 (ref. 57) | 18.2 |
| This work | 0.5 | 110 | 93 | 186 |
The TON for the formation of compound I is observed to be 186 with our catalytic method, whereas it is 18.2 by utilising the method of Uchiyama and co-workers (Table 3). Hence, our method utilising 2 and 5 as the aryl transfer reagent works much more efficiently, leading to scope for future exploration of more NHSi-coordinated organocopper compounds in various other organic transformations. This enhanced catalytic performance is presumably due to the strong σ-donor properties of NHSi, which are also observed in other previous reports.1c
3 Conclusions
In summary, we have established a versatile route to utilise the NHSi–organocoinage metal complexes as an efficient precursor for heteroatom functionalisation. The derivatisation of NHC or CAAC-based coinage metal complexes has become important lately because of the efficient optoelectronic applications of carbene–metal–amide complexes and several organic transformations. However, the functionalisation of NHSi–Cu(I) and Ag(I) complexes was unknown to date. Our adopted methodology allows facile formation of NHSi-supported Cu–B (12), Cu/Ag–Se (14–16), Cu/Ag–N (17–22), and Cu/Ag–P (23 and 24) bonds. Moreover, utilising N-donor attached secondary amines gives three-coordinate copper(I) complexes (21 and 22). This synthetic protocol helped us to isolate rarely occurring coinage metal phosphides, 23 and 24. We have also demonstrated the potential of these NHSi–organocoinage metal complexes in two important catalytic organic transformations, which led to the isolation of desired products in excellent yields, paving the way for more exploration in this area.Data availability
The data supporting this article have been included as part of the ESI.† The ESI contains experimental data, X-ray data, NMR spectra, computational details, and related references.Author contributions
S. K. and M. G. conceived the idea of the project. M. G. contributed to the design, development, execution, and compilation of the project. M. G. performed all the syntheses and characterisation studies and wrote the original draft with the help of P. P. P. P. performed all the theoretical calculations, prepared the corresponding figures, and provided insightful feedback. K. G. carried out catalytic reactions and curated spectroscopic details in the ESI,† and prepared the figures of characterisation methods along with M. G. S. T. refined the crystallographic data provided by M. G. R. K. P. put significant efforts into refining additional crystal data during the revision. All authors contributed to the discussion. S. K. contributed to fund acquisition, project administration, offered critical insights, coordinated the research, and finalised the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
S. K. thanks SERB-CRG for the financial support. S. K. also thanks DST-FIST for a single-crystal X-ray diffractometer facility. M. G. and P. P. thank IISER Pune for providing a fellowship. K. G. acknowledges the Prime Minister Research Fellowship (PMRF) for providing a fellowship. S. T. is grateful to AESD&CIF and CSIR-CSMCRI. M.G. thanks Dr Sandeep Mishra for his insightful help and discussion regarding NMR. We express our gratitude for the support and resources extended by the “PARAM Brahma Facility” under the National Supercomputing Mission, Government of India, located at IISER Pune.Notes and references
- (a) B. Blom, D. Gallego and M. Driess, Inorg. Chem. Front., 2014, 1, 134–148 RSC; (b) B. Blom, M. Stoelzel and M. Driess, Chem.–Eur. J., 2013, 19, 40–62 CrossRef CAS PubMed; (c) Y. P. Zhou and M. Driess, Angew. Chem., Int. Ed., 2019, 58, 3715–3728 CrossRef CAS PubMed; (d) S. Yao, A. Saddington, Y. Xiong and M. Driess, Acc. Chem. Res., 2023, 56, 475–488 CrossRef CAS PubMed; (e) L. Wang, Y. Li, Z. Li and M. Kira, Coord. Chem. Rev., 2022, 457, 214413–214441 CrossRef CAS; (f) S. S. Sen, S. Khan, P. P. Samuel and H. W. Roesky, Chem. Sci., 2012, 3, 659–682 RSC; (g) M. Haaf, T. A. Schmedake and R. West, Acc. Chem. Res., 2000, 33, 704–714 CrossRef CAS PubMed; (h) S. Takahashi, E. Bellan, A. Baceiredo, N. Saffon-Merceron, S. Massou, N. Nakata, D. Hashizume, V. Branchadell and T. Kato, Angew. Chem., Int. Ed., 2019, 58, 10310–10314 CrossRef CAS PubMed; (i) I. Alvarado-Beltran, A. Baceiredo, N. Saffon-Merceron, V. Branchadell and T. Kato, Angew. Chem., Int. Ed., 2016, 55, 16141–16144 CrossRef CAS PubMed.
- C. K. Shan, S. L. Yao and M. Driess, Chem. Soc. Rev., 2020, 49, 6733–6754 RSC.
- (a) D. Gallego, A. Brück, E. Irran, F. Meier, M. Kaupp, M. Driess and J. F. Hartwig, J. Am. Chem. Soc., 2013, 135, 15617–15626 CrossRef CAS PubMed; (b) Y.-P. Zhou, S. Raoufmoghaddam, T. Szilvási and M. Driess, Angew. Chem., Int. Ed., 2016, 55, 12868–12872 CrossRef CAS PubMed.
- (a) Y. Wang, M. Karni, S. Yao, A. Kaushansky, Y. Apeloig and M. Driess, J. Am. Chem. Soc., 2019, 141, 12916–12927 CrossRef CAS PubMed; (b) Y. Wang, T. Szilvási, S. Yao and M. Driess, Nat. Chem., 2020, 12, 801–807 CrossRef CAS PubMed; (c) M. P. Luecke, L. Giarrana, A. Kostenko, T. Gensch, S. Yao and M. Driess, Angew. Chem., Int. Ed., 2022, 61, e202110398 CrossRef CAS PubMed; (d) Y. Xiong, S. Dong, S. Yao, J. Zhu and M. Driess, Angew. Chem., Int. Ed., 2022, 61, e202205358 CrossRef CAS PubMed.
- (a) P. Jutzi, U. Holtmann, D. Kanne, C. Krüger, R. Blom, R. Gleiter and I. Hyla-Kryspin, Chem. Ber., 1989, 122, 1629–1639 CrossRef CAS; (b) M. Denk, R. Lennon, R. Hayashi, R. West, A. V. Belyakov, H. P. Verne, A. Haaland, M. Wagner and N. Metzler, J. Am. Chem. Soc., 1994, 116, 2691–2692 CrossRef CAS; (c) C. W. So, H. W. Roesky, J. Magull and R. B. Oswald, Angew. Chem., Int. Ed., 2006, 45, 3948–3950 CrossRef CAS PubMed; (d) M. Kira, S. Ishida, T. Iwamoto and C. Kabuto, J. Am. Chem. Soc., 1999, 121, 9722–9723 CrossRef CAS; (e) M. Asay, S. Inoue and M. Driess, Angew. Chem., Int. Ed., 2011, 50, 9589–9592 CrossRef CAS PubMed; (f) B. D. Rekken, T. M. Brown, J. C. Fettinger, H. M. Tuononen and P. P. Power, J. Am. Chem. Soc., 2012, 134, 6504–6507 CrossRef CAS PubMed; (g) A. V. Protchenko, K. H. Birjkumar, D. Dange, A. D. Schwarz, D. Vidovic, C. Jones, N. Kaltsoyannis, P. Mountford and S. Aldridge, J. Am. Chem. Soc., 2012, 134, 6500–6503 CrossRef CAS PubMed; (h) A. Rosas-Sanchez, I. Alvarado-Beltran, A. Baceiredo, N. Saffon-Merceron, S. Massou, V. Branchadell and T. Kato, Angew. Chem., Int. Ed., 2017, 56, 10549–10554 CrossRef CAS PubMed; (i) S. Fujimori and S. Inoue, Eur. J. Inorg. Chem., 2020, 2020, 3131–3142 CrossRef CAS PubMed; (j) R. Guthardt and C. Jones, Chem. Commun., 2024, 60, 1583–1586 RSC; (k) M. M. Roy, M. J. Ferguson, R. McDonald, Y. Zhou and E. Rivard, Chem. Sci., 2019, 10, 6476–6481 RSC; (l) P. Garg, A. Carpentier, I. Douair, D. Dange, Y. Jiang, K. Yuvaraj, L. Maron and C. Jones, Angew. Chem., Int. Ed., 2022, 61, e202201705 CrossRef CAS PubMed.
- (a) M. Ghosh and S. Khan, Dalton Trans., 2021, 50, 10674–10688 RSC; (b) C. Weetman and S. Inoue, ChemCatChem, 2018, 10, 4213–4228 CrossRef CAS; (c) H. Zhu, A. Kostenko, D. Franz, F. Hanusch and S. Inoue, J. Am. Chem. Soc., 2023, 145, 1011–1021 CrossRef CAS PubMed; (d) D. Wendel, D. Reiter, A. Porzelt, P. J. Altmann, S. Inoue and B. Rieger, J. Am. Chem. Soc., 2017, 139, 17193–17198 CrossRef CAS PubMed.
- (a) J. Hossain, J. S. Gopinath, S. Tothadi, P. Parameswaran and S. Khan, Organometallics, 2022, 41, 3706–3717 CrossRef CAS; (b) M. Ghosh and S. Khan, in Advances in Inorganic Chemistry, Elsevier, 2023, vol. 81, pp. 279–303 Search PubMed; (c) M. Ghosh, S. Tothadi and S. Khan, Organometallics, 2021, 40, 3201–3210 CrossRef CAS; (d) N. Parvin, N. Sen, S. Tothadi, S. Muhammed, P. Parameswaran and S. Khan, Organometallics, 2021, 40, 1626–1632 CrossRef CAS; (e) N. Parvin, J. Hossain, A. George, P. Parameswaran and S. Khan, Chem. Commun., 2020, 56, 273–276 RSC; (f) N. Parvin, B. Mishra, A. George, M. Neralkar, J. Hossain, P. Parameswaran, S. Hotha and S. Khan, Chem. Commun., 2020, 56, 7625–7628 RSC; (g) N. Parvin, S. Pal, J. Echeverria, S. Alvarez and S. Khan, Chem. Sci., 2018, 9, 4333–4337 RSC; (h) M. Nazish, H. Bai, C. M. Legendre, R. Herbst-Irmer, L. Zhao, D. Stalke and H. W. Roesky, Chem. Commun., 2022, 58, 12704–12707 RSC; (i) S. Khan, S. Pal, N. Kathewad, I. Purushothaman, S. De and P. Parameswaran, Chem. Commun., 2016, 52, 3880–3882 RSC; (j) R. Yadav, X. Sun, R. Köppe, M. T. Gamer, F. Weigend and P. W. Roesky, Angew. Chem., Int. Ed., 2022, 61, e202211115 CrossRef CAS PubMed.
- (a) W. Xie and S. Chang, Angew. Chem., Int. Ed., 2016, 55, 1876–1880 CrossRef CAS PubMed; (b) A. Bonet, V. Lillo, J. Ramírez, M. M. Díaz-Requejo and E. Fernández, Org. Biomol. Chem., 2009, 7, 1533–1535 RSC; (c) T. Ohishi, M. Nishiura and Z. Hou, Angew. Chem., Int. Ed., 2008, 47, 5792–5795 CrossRef CAS PubMed; (d) J. R. Herron and Z. T. Ball, J. Am. Chem. Soc., 2008, 130, 16486–16487 CrossRef CAS PubMed; (e) L. Zhang, Z. Li, M. Takimoto and Z. Hou, Chem. Rec., 2020, 20, 494–512 CrossRef CAS PubMed; (f) S. Bagherzadeh and N. P. Mankad, J. Am. Chem. Soc., 2015, 137, 10898–10901 CrossRef CAS PubMed; (g) L. E. English, T. M. H. Downie, C. L. Lyall, M. F. Mahon, C. L. McMullin, S. E. Neale, C. M. Saunders and D. J. Liptrot, Chem. Commun., 2023, 59, 1074–1077 RSC; (h) T. M. Horsley Downie, M. F. Mahon, J. P. Lowe, R. M. Bailey and D. J. Liptrot, ACS Catal., 2022, 12, 8214–8219 CrossRef CAS; (i) T. M. H. Downie, J. W. Hall, T. P. C. Finn, D. J. Liptrot, J. P. Lowe, M. F. Mahon, C. L. McMullin and M. K. Whittlesey, Chem. Commun., 2020, 56, 13359–13362 RSC; (j) S. Ostrowska, P. Arnaut, D. J. Liptrot, C. S. Cazin and S. P. Nolan, Chem. Commun., 2023, 59, 9126–9129 RSC; (k) L. Zhang and Z. Hou, Chem. Sci., 2013, 4, 3395–3403 RSC; (l) F. Lazreg, F. Nahra and C. S. J. Cazin, Coord. Chem. Rev., 2015, 293, 48–79 CrossRef; (m) J. Hossain, R. Akhtar and S. Khan, Polyhedron, 2021, 201, 115151–115187 CrossRef CAS; (n) S. Díez-González, E. D. Stevens, N. M. Scott, J. L. Petersen and S. P. Nolan, Chem.–Eur. J., 2008, 14, 158–168 CrossRef PubMed; (o) L. Yang and H. Wang, ChemSusChem, 2014, 7, 962–998 CrossRef CAS PubMed; (p) A. A. Danopoulos, T. Simler and P. Braunstein, Chem. Rev., 2019, 119, 3730–3961 CrossRef CAS PubMed.
- J. C. Garrison and W. J. Youngs, Chem. Rev., 2005, 105, 3978–4008 CrossRef CAS PubMed.
- M. Stollenz and F. Meyer, Organometallics, 2012, 31, 7708–7727 CrossRef CAS.
- (a) A. G. Avent, B. Gehrhus, P. B. Hitchcock, M. F. Lappert and H. Maciejewski, J. Organomet. Chem., 2003, 686, 321–331 CrossRef CAS; (b) Y. Inagawa, S. Ishida and T. Iwamoto, Chem. Lett., 2014, 43, 1665–1667 CrossRef CAS; (c) G. Tan, B. Blom, D. Gallego, E. Irran and M. Driess, Chem.–Eur. J., 2014, 20, 9400–9408 CrossRef CAS PubMed; (d) G. Tan, B. Blom, D. Gallego and M. Driess, Organometallics, 2014, 33, 363–369 CrossRef CAS; (e) T. Troadec, A. Prades, R. Rodriguez, R. Mirgalet, A. Baceiredo, N. Saffon-Merceron, V. Branchadell and T. Kato, Inorg. Chem., 2016, 55, 8234–8240 CrossRef CAS PubMed; (f) N. Parvin, R. Dasgupta, S. Pal, S. S. Sen and S. Khan, Dalton Trans., 2017, 46, 6528–6532 RSC; (g) N. Parvin, S. Pal, S. Khan, S. Das, S. K. Pati and H. W. Roesky, Inorg. Chem., 2017, 56, 1706–1712 CrossRef CAS PubMed; (h) S. Abe, Y. Inagawa, R. Kobayashi, S. Ishida and T. Iwamoto, Organometallics, 2022, 41, 874–882 CrossRef CAS; (i) M. Nazish, M. M. Siddiqui, S. K. Sarkar, A. Munch, C. M. Legendre, R. Herbst-Irmer, D. Stalke and H. W. Roesky, Chem.–Eur. J., 2021, 27, 1744–1752 CrossRef CAS PubMed.
- S. Amanullah, W. Cao, E. Brack, M. Plodinec and C. Copéret, Angew. Chem., Int. Ed., 2025, 64, e202417428 CrossRef CAS PubMed.
- (a) D. S. Laitar, P. Müller and J. P. Sadighi, J. Am. Chem. Soc., 2005, 127, 17196–17197 CrossRef CAS PubMed; (b) Y. Segawa, M. Yamashita and K. Nozaki, Angew. Chem., Int. Ed., 2007, 46, 6710–6713 CrossRef CAS PubMed; (c) R. S. Charman, J. A. Hobson, R. A. Jackson, M. F. Mahon, S. E. Neale and D. J. Liptrot, Chem.–Eur. J., 2024, 30, e202302704 CrossRef CAS PubMed; (d) D. J. Babula, R. S. Charman, T. H. Jerome, T. M. Horsley Downie, M. F. Mahon and D. J. Liptrot, Eur. J. Inorg. Chem., 2023, 26, e202300043 CrossRef CAS; (e) W. Drescher, C. Borner and C. Kleeberg, New J. Chem., 2021, 45, 14957–14964 RSC.
- (a) A. S. Romanov, C. R. Becker, C. E. James, D. Di, D. Credgington, M. Linnolahti and M. Bochmann, Chem.–Eur. J., 2017, 23, 4625–4637 CrossRef CAS PubMed; (b) W. Zou, Q. Zhu, J. C. Fettinger and P. P. Power, Inorg. Chem., 2021, 60, 17641–17648 CrossRef CAS PubMed; (c) M. Azizpoor Fard, A. M. Polgar and J. F. Corrigan, Organometallics, 2020, 39, 2900–2906 CrossRef CAS; (d) J. Kampf, R. Kumar and J. P. Oliver, Inorg. Chem., 1992, 31, 3626–3629 CrossRef CAS.
- M. Ghosh, J. Chatterjee, P. Panwaria, A. Kudlu, S. Tothadi and S. Khan, Angew. Chem., Int. Ed., 2024, 63, e202410792 CrossRef CAS PubMed.
- (a) R. Jazzar, M. Soleilhavoup and G. Bertrand, Chem. Rev., 2020, 120, 4141–4168 CrossRef CAS PubMed; (b) R. Hamze, J. L. Peltier, D. Sylvinson, M. Jung, J. Cardenas, R. Haiges, M. Soleilhavoup, R. Jazzar, P. I. Djurovich, G. Bertrand and M. E. Thompson, Science, 2019, 363, 601–606 CrossRef CAS PubMed; (c) P. J. Conaghan, C. S. B. Matthews, F. Chotard, S. T. E. Jones, N. C. Greenham, M. Bochmann, D. Credgington and A. S. Romanov, Nat. Commun., 2020, 11, 1758 CrossRef CAS PubMed; (d) M. Gernert, L. Balles-Wolf, F. Kerner, U. Müller, A. Schmiedel, M. Holzapfel, C. M. Marian, J. Pflaum, C. Lambert and A. Steffen, J. Am. Chem. Soc., 2020, 142, 8897–8909 CrossRef CAS PubMed; (e) A. S. Romanov, S. T. Jones, Q. Gu, P. J. Conaghan, B. H. Drummond, J. Feng, F. Chotard, L. Buizza, M. Foley, M. Linnolahti, D. Credgington and M. Bochmann, Chem. Sci., 2020, 11, 435–446 RSC; (f) R. Tang, S. Xu, T.-L. Lam, G. Cheng, L. Du, Q. Wan, J. Yang, F.-F. Hung, K.-H. Low, D. L. Phillips and C.-M. Che, Angew. Chem., Int. Ed., 2022, 61, e202203982 CrossRef CAS PubMed.
- (a) T. Tsuda, T. Yazawa, K. Watanabe, T. Fujii and T. Saegusa, J. Org. Chem., 1981, 46, 192–194 CrossRef CAS; (b) S. Gambarotta, C. Floriani, A. Chiesi-Villa and C. Guastini, J. Chem. Soc., Chem. Commun., 1983, 1156–1158 RSC; (c) E. M. Meyer, S. Gambarotta, C. Floriani, A. Chiesi-Villa and C. Guastini, Organometallics, 1989, 8, 1067–1079 CrossRef CAS; (d) S. S. Sen, J. Hey, R. Herbst-Irmer, H. W. Roesky and D. Stalke, J. Am. Chem. Soc., 2011, 133, 12311–12316 CrossRef CAS PubMed.
- C. Boehme and G. Frenking, Organometallics, 1998, 17, 5801–5809 CrossRef CAS.
- L. Kuehn, A. F. Eichhorn, D. Schmidt, T. B. Marder and U. Radius, J. Organomet. Chem., 2020, 919, 121249 CrossRef CAS.
- M. Riethmann, S. A. Föhrenbacher, H. Keiling, N. V. Ignat’ev, M. Finze and U. Radius, Inorg. Chem., 2024, 63, 8351–8365 CrossRef CAS PubMed.
- M. Dahlen, T. P. Seifert, S. Lebedkin, M. T. Gamer, M. M. Kappes and P. W. Roesky, Chem. Commun., 2021, 57, 13146–13149 RSC.
- H. Schmidbaur and A. Schier, Angew. Chem., Int. Ed., 2015, 54, 746–784 CrossRef CAS PubMed.
- B. K. Tate, A. J. Jordan, J. Bacsa and J. P. Sadighi, Organometallics, 2017, 36, 964–974 CrossRef CAS.
- Y. Liu, L. J. Taylor, S. P. Argent, J. McMaster and D. L. Kays, Inorg. Chem., 2021, 60, 10114–10123 CrossRef CAS PubMed.
- B. K. Tate, C. M. Wyss, J. Bacsa, K. Kluge, L. Gelbaum and J. P. Sadighi, Chem. Sci., 2013, 4, 3068–3074 RSC.
- D. Li, I. Keresztes, R. Hopson and P. G. Williard, Acc. Chem. Res., 2009, 42, 270–280 CrossRef CAS PubMed.
- (a) C. M. Lemon, D. C. Powers, M. Huynh, A. G. Maher, A. A. Phillips, B. P. Tripet and D. G. Nocera, Inorg. Chem., 2022, 62, 3–17 CrossRef PubMed; (b) M. P. Carranza, B. R. Manzano, F. A. Jalón, A. M. Rodriguez, L. Santos and M. Moreno, New J. Chem., 2013, 37, 3183–3194 RSC; (c) M. P. Carranza, B. R. Manzano, F. A. Jalon, A. M. Rodriguez, L. Santos and M. Moreno, Inorg. Chem., 2010, 49, 3828–3835 CrossRef CAS PubMed; (d) L. Ray, M. M. Shaikh and P. Ghosh, Inorg. Chem., 2008, 47, 230–240 CrossRef CAS PubMed; (e) J. E. Aguado, M. J. Calhorda, P. J. Costa, O. Crespo, V. Félix, M. C. Gimeno, P. G. Jones and A. Laguna, Eur. J. Inorg. Chem., 2004, 2004, 3038–3047 CrossRef.
- (a) S. S. Sen, H. W. Roesky, D. Stern, J. Henn and D. Stalke, J. Am. Chem. Soc., 2010, 132, 1123–1126 CrossRef CAS PubMed; (b) R. Azhakar, R. S. Ghadwal, H. W. Roesky, H. Wolf and D. Stalke, Organometallics, 2012, 31, 4588–4592 CrossRef CAS.
- R. Waterman, Organometallics, 2013, 32, 7249–7263 CrossRef CAS.
- (a) K. K. Hollister, A. Molino, G. Breiner, J. E. Walley, K. E. Wentz, A. M. Conley, D. A. Dickie, D. J. Wilson and R. J. Gilliard Jr, J. Am. Chem. Soc., 2022, 144, 590–598 CrossRef CAS PubMed; (b) K. E. Wentz, A. Molino, L. A. Freeman, D. A. Dickie, D. J. Wilson and R. J. Gilliard Jr, J. Am. Chem. Soc., 2022, 144, 16276–16281 CrossRef CAS PubMed; (c) S. K. Sarkar, K. K. Hollister, A. Molino, A. D. Obi, C.-L. Deng, B. Y. E. Tra, B. M. Stewart, D. A. Dickie, D. J. D. Wilson and R. J. Gilliard Jr, J. Am. Chem. Soc., 2023, 145, 21475–21482 CrossRef CAS PubMed.
- (a) P. Bissinger, A. Steffen, A. Vargas, R. D. Dewhurst, A. Damme and H. Braunschweig, Angew. Chem., Int. Ed., 2015, 54, 4362–4366 CrossRef CAS PubMed; (b) H. Braunschweig, T. Dellermann, R. D. Dewhurst, B. Hupp, T. Kramer, J. D. Mattock, J. Mies, A. K. Phukan, A. Steffen and A. Vargas, J. Am. Chem. Soc., 2017, 139, 4887–4893 CrossRef CAS PubMed.
- (a) E. C. Neeve, S. J. Geier, I. A. Mkhalid, S. A. Westcott and T. B. Marder, Chem. Rev., 2016, 116, 9091–9161 CrossRef CAS PubMed; (b) R. D. Dewhurst, E. C. Neeve, H. Braunschweig and T. B. Marder, Chem. Commun., 2015, 51, 9594–9607 RSC.
- A. F. Eichhorn, L. Kuehn, T. B. Marder and U. Radius, Chem. Commun., 2017, 53, 11694–11696 RSC.
- L. Dang, H. Zhao, Z. Lin and T. B. Marder, Organometallics, 2008, 27, 1178–1186 CrossRef CAS.
- (a) C. M. Wyss, J. Bitting, J. Bacsa, T. G. Gray and J. P. Sadighi, Organometallics, 2016, 35, 71–74 CrossRef CAS; (b) C. Kleeberg and C. Borner, Organometallics, 2018, 37, 4136–4146 CrossRef CAS; (c) C. Borner, L. Anders, K. Brandhorst and C. Kleeberg, Organometallics, 2017, 36, 4687–4690 CrossRef CAS; (d) P. M. Rutz, J. r. Grunenberg and C. Kleeberg, Organometallics, 2022, 41, 3044–3054 CrossRef CAS; (e) P. Ríos, M. S. See, R. C. Handford, S. J. Teat and T. D. Tilley, Chem. Sci., 2022, 13, 6619–6625 RSC.
- G. A. Molander, S. L. Trice and S. D. Dreher, J. Am. Chem. Soc., 2010, 132, 17701–17703 CrossRef CAS.
- N. Oka, T. Yamada, H. Sajiki, S. Akai and T. Ikawa, Org. Lett., 2022, 24, 3510–3514 CrossRef CAS PubMed.
- R. An, L. Liao, X. Liu, S. Song and X. Zhao, Org. Chem. Front., 2018, 5, 3557–3561 RSC.
- S. Kolay, A. Wadawale, D. Das, H. K. Kisan, R. B. Sunoj and V. K. Jain, Dalton Trans., 2013, 42, 10828–10837 RSC.
- W. J. Humenny, S. Mitzinger, C. B. Khadka, B. K. Najafabadi, I. Vieira and J. F. Corrigan, Dalton Trans., 2012, 41, 4413–4422 RSC.
- N. V. S. Harisomayajula, S. Makovetskyi and Y. C. Tsai, Chem.–Eur. J., 2019, 25, 8936–8954 CrossRef CAS PubMed.
- R. Bühler, R. M. Wolf, C. Gemel, J. Stephan, S. N. Deger, S. Kahlal, R. A. Fischer and J.-Y. Saillard, Inorg. Chem., 2024, 63, 17617–17625 CrossRef PubMed.
- (a) M. Stollenz, M. John, H. Gehring, S. Dechert, C. Grosse and F. Meyer, Inorg. Chem., 2009, 48, 10049–10059 CrossRef CAS PubMed; (b) J. I. van der Vlugt, Chem. Soc. Rev., 2010, 39, 2302–2322 RSC; (c) P. A. Chase and D. W. Stephan, Angew. Chem., Int. Ed., 2008, 47, 7433–7437 CrossRef CAS PubMed.
- (a) T. Scattolin, A. A. Logvinov, N. V. Tzouras, C. S. J. Cazin and S. P. Nolan, Organometallics, 2023, 42, 2692–2730 CrossRef CAS; (b) L. A. Goj, E. D. Blue, C. Munro-Leighton, T. B. Gunnoe and J. L. Petersen, Inorg. Chem., 2005, 44, 8647–8649 CrossRef CAS PubMed; (c) L. A. Goj, E. D. Blue, S. A. Delp, T. B. Gunnoe, T. R. Cundari, A. W. Pierpont, J. L. Petersen and P. D. Boyle, Inorg. Chem., 2006, 45, 9032–9045 CrossRef CAS.
- R. Molteni, K. Edkins, M. Haehnel and A. Steffen, Organometallics, 2016, 35, 629–640 CrossRef CAS.
- V. A. Krylova, P. I. Djurovich, B. L. Conley, R. Haiges, M. T. Whited, T. J. Williams and M. E. Thompson, Chem. Commun., 2014, 50, 7176–7179 RSC.
- A. N. Paesch, A. K. Kreyenschmidt, R. Herbst-Irmer and D. Stalke, Inorg. Chem., 2019, 58, 7000–7009 CrossRef CAS PubMed.
- S. T. Barry, Coord. Chem. Rev., 2013, 257, 3192–3201 CrossRef CAS.
- A. S. Romanov, F. Chotard, J. Rashid and M. Bochmann, Dalton Trans., 2019, 48, 15445–15454 RSC.
- F. Chotard, V. Sivchik, M. Linnolahti, M. Bochmann and A. S. Romanov, Chem. Mater., 2020, 32, 6114–6122 CrossRef CAS.
- J. Kögel, D. Sorokin, A. Khvorost, M. Scott, K. Harms, D. Himmel, I. Krossing and J. Sundermeyer, Chem. Sci., 2018, 9, 245–253 RSC.
- B. K. Najafabadi and J. F. Corrigan, Dalton Trans., 2015, 44, 14235–14241 RSC.
- B. K. Najafabadi and J. F. Corrigan, Chem. Commun., 2015, 51, 665–667 RSC.
- (a) G. C. Fortman, A. M. Slawin and S. P. Nolan, Organometallics, 2010, 29, 3966–3972 CrossRef CAS; (b) J. Yuan, L. Zhu, J. Zhang, J. Li and C. Cui, Organometallics, 2017, 36, 455–459 CrossRef CAS.
- (a) H. Ahuja, H. Kaur and R. Arevalo, Inorg. Chem. Front., 2023, 10, 6067–6076 RSC; (b) S. Kalra, D. Pividori, D. Fehn, C. Dai, S. Dong, S. Yao, J. Zhu, K. Meyer and M. Driess, Chem. Sci., 2022, 13, 8634–8641 RSC.
- (a) L. Yin and J. Liebscher, Chem. Rev., 2007, 107, 133–173 CrossRef CAS; (b) M. Busch, M. D. Wodrich and C. Corminboeuf, ACS Catal., 2017, 7, 5643–5653 CrossRef CAS.
- M. Oi, R. Takita, J. Kanazawa, A. Muranaka, C. Wang and M. Uchiyama, Chem. Sci., 2019, 10, 6107–6112 RSC.
- (a) W. Wang, S. Inoue, S. Yao and M. Driess, J. Am. Chem. Soc., 2010, 132, 15890–15892 CrossRef CAS PubMed; (b) C. I. Someya, M. Haberberger, W. Wang, S. Enthaler and S. Inoue, Chem. Lett., 2013, 42, 286–288 CrossRef CAS.
- R. Martin and S. L. Buchwald, Acc. Chem. Res., 2008, 41, 1461–1473 CrossRef CAS PubMed.
- J. Wang, X. Liu and X. Feng, Chem. Rev., 2011, 111, 6947–6983 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2304275 (2), 2304278 (3), 2419144 (4), 2450951 (5), 2304292 (6), 2419145 (7), 2419147 (8), 2304280 (9), 2450954 (10), 2450956 [PhC{N(tBu)}2Si{N(Dipp)SiMe3}], 2450959 (11), 2450960 (12), 2304289 (13), 2304286 (14), 2325641 (15), 2419148 (16), 2450961 (17), 2304281 (18), 2304284 (19), 2304285 (20), 2304283 (21), 2419162 (22), 2419149 (23) and 2419163 (24). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5sc00879d |
| ‡ Both authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |