Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of γ-alkylidene lactones via molecular stitching of carboxylic acids and olefins†
Edis
Crnovrsanin‡
,
Sourjya
Mal‡
and
Manuel
van Gemmeren
 *
*
Otto Diels-Institut für Organische Chemie, Christian-Albrechts Universität zu Kiel, Otto-Hahn-Platz 4, Kiel, 24118, Germany. E-mail: vangemmeren@oc.uni-kiel.de
First published on 4th July 2025
Abstract
In this study, we describe a direct palladium-catalyzed tandem β-C(sp3)–H olefination/lactonization strategy for the one-step synthesis of γ-alkylidene lactones. The reaction is enabled by an N-acyl sulfonamide ligand and utilizes readily available and inexpensive carboxylic acids and styrenes as coupling partners, enabling the efficient construction of structurally diverse γ-alkylidene lactones through the consecutive functionalization of three C–H bonds. Notable highlights include excellent functional group tolerance and derivatization of the resulting γ-alkylidene lactones to obtain various synthetically useful motifs.
Introduction
γ-Alkylidene lactones represent an important structural unit due to their widespread occurrence in various classes of natural products1 and diverse biological activities.2 Besides the great potential of these scaffolds to be used as a synthetic linchpin, their utilization in asymmetric synthesis has received considerable attention.3 Consequently, substantial efforts have been devoted towards their synthesis, which typically rely on Lewis acid-catalyzed cyclization reactions of acetylenic acids.4 However, the multistep synthetic sequences required for the preparation of the acetylenic acid precursors limit their broader application. A more general and practical approach would be to access γ-alkylidene lactones in one step from the commercially available feedstock chemicals, enabling an atom- and step-economic synthesis. Based on our previous experience in the area of carboxylic acid directed C(sp3)–H activation reactions,5 we envisioned that they could, in principle, be made by molecular stitching6 of olefins and carboxylic acids via a triple C–H functionalization strategy (Scheme 1B), which could be achieved by a sequence of carboxylic acid directed β-C(sp3)–H activation/olefination and subsequent cyclization via carboxylic acid directed vinylic C(sp2)–H activation/lactonization. Notably, this strategy differs fundamentally from the previously reported reactivity of carboxylic acids with activated olefins (i.e. acrylates, vinyl sulfones, vinyl phosphonates, etc.) and allylic electrophiles, in which the initially formed olefinated acid tends to undergo subsequent cyclization via intramolecular Michael addition, resulting in the formation of γ-lactones (Scheme 1C).7 Further motivation to pursue this strategy came from the description of a somewhat analogous reactivity with amides and protected amines8 as substrates (Scheme 1C).9 Therefore, building upon our recent contributions to carbon–heteroatom bond forming reactions,10 we herein report the development of a protocol for the direct synthesis of a wide range of γ-alkylidene lactones from readily available and inexpensive aliphatic carboxylic acids and styrenes via the one-pot functionalization of three C–H bonds.Results and discussion
We commenced our studies using 2,2-dimethyl butyric acid (1b) as a model substrate and styrene (2a) as a coupling partner. Given the poor reactivity of styrenes in the analogous olefination reactions of free carboxylic acids,7a–d we anticipated that the choice of a suitable ligand scaffold would be essential for promoting the particularly challenging carbopalladation step alongside other key steps of the catalytic cycle. Consequently, a comprehensive initial screening of ligands (see the ESI† for more details) led to the discovery of the N-acyl sulfonamide (NASA) ligand L10 as a particularly promising ligand, and we optimized the reaction conditions using this ligand. After identifying the best reaction conditions, we re-evaluated common ligand classes (Scheme 2). As expected, only traces of product (3b) were detected in the absence of ligand, indicating the crucial role of the external ligand in achieving the desired reactivity. We observed that amino acid-derived ligand (L1, 24%)11 and ethylenediamine-based ligands12 (L2, 29% and L3, 10%) continued to deliver unsatisfying results. Next, a series of N-acetyl thioether ligands,7a the second-best ligand class in the initial screening, was tested (L4, 38%; L5, 42%; and L6, 37%), delivering improved results, albeit with still moderate yields. Due to the well-known isosteric relationship between the sulfonamide moiety and carboxylic acid,13 we hypothesized that replacing the carboxylic acid donor with a sulfonamide would not only allow for fine-tuning of the pKa of the donor group, but also enable the introduction of steric bulk near the donor atom. Taking this into consideration and prompted by our recent study in which NASA-ligands were shown to be highly effective for Pd-catalyzed C(sp2)–H deuteration14 and the success of these ligands in challenging C(sp3)–H functionalizations,15 a range of NASA-ligands with electronically different substituents on the phenyl ring was examined. While electron-withdrawing substituents on the phenyl ring (L7, 29%) continued to offer moderate yield, electron-donating substituents (L8, 39% and L9, 40%) improved the reaction outcome. The catalytic efficiency was strongly enhanced by the introduction of a 2,4,6-triisopropyl group (L10, 58%), presumably due to the changes in the sulfonamide geometry caused by the steric bulk. Various substituents on the backbone (L11–L14) resulted in decreased yields. Furthermore, switching to a different concerted metalation–deprotonation (CMD)-promoting amide group (L15, 36%) gave no further improvement. Lastly, a series of co-oxidants16 was tested (see the ESI† for more details) in combination with the optimal ligand L10. Gratifyingly, the addition of MnO2 (20 mol%) proved beneficial, affording 3b in 66% yield.Having identified suitable reaction conditions, we first explored the scope of aliphatic acid substrates. As expected from the optimization studies, the desired lactone derived from model substrate 1b was obtained in 65% (3b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3b′ = 10
3b′ = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Scheme 3) isolated yield. Aliphatic acids with varying chain lengths and branching patterns on the side chain afforded their respective γ-alkylidene lactones in satisfactory yields, including methyl (45%, 3a
1, Scheme 3) isolated yield. Aliphatic acids with varying chain lengths and branching patterns on the side chain afforded their respective γ-alkylidene lactones in satisfactory yields, including methyl (45%, 3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a′ = 11
3a′ = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), n-butyl (47%, 3c
1), n-butyl (47%, 3c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3c′ = 6
3c′ = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and isobutyl (3d, 50%) substituted products. Substrates bearing a five- (47%, 3e
1), and isobutyl (3d, 50%) substituted products. Substrates bearing a five- (47%, 3e![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3e′ = 20
3e′ = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) or six- (3f, 52%) membered ring in the side chain were well-tolerated. Interestingly, 1g, which typically tends to undergo dehydrogenation via functionalization of methylene C(sp3)–H bonds,17 afforded 3g (70%) in excellent yield. Additionally, substrates having halogens (3h, 56% and 3i, 44%), trifluoromethyl (3j, 42%), or a protected hydroxyl group (3k, 35%) on the side chain were well-tolerated by our protocol. We then tested 4-aryl-substituted aliphatic acids featuring reactive benzylic C(sp3)–H bonds with electronically diverse arenes. While 1l bearing simple phenyl afforded the respective lactone in 50% (3l
1) or six- (3f, 52%) membered ring in the side chain were well-tolerated. Interestingly, 1g, which typically tends to undergo dehydrogenation via functionalization of methylene C(sp3)–H bonds,17 afforded 3g (70%) in excellent yield. Additionally, substrates having halogens (3h, 56% and 3i, 44%), trifluoromethyl (3j, 42%), or a protected hydroxyl group (3k, 35%) on the side chain were well-tolerated by our protocol. We then tested 4-aryl-substituted aliphatic acids featuring reactive benzylic C(sp3)–H bonds with electronically diverse arenes. While 1l bearing simple phenyl afforded the respective lactone in 50% (3l![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3l′ = 10
3l′ = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) yield, electron-deficient arene improved the yield to 60% (3m
1) yield, electron-deficient arene improved the yield to 60% (3m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3m′ = 20
3m′ = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), implying that the reaction is sensitive to the electronic properties of the aryl group at this position. Next, we evaluated a series of 3-aryl-substituted aliphatic acids featuring equidistant benzylic C–H bonds, the activation of which would trigger side reactions/decomposition pathways, rendering these substrates particularly challenging. Notably, besides the simple phenyl group (3n, 40%), a range of synthetically useful functional groups, including an ester (3o, 41%), a nitro (3p, 43%), a trifluoromethylthio (3q, 44%), and a trifluoromethyl (3r, 45%) group, were found to be amenable with our protocol. Furthermore, phenyl acetic acid-derived 1s, in which ortho C(sp2)–H activation/functionalization competes with the desired reactivity,18 afforded 3s, albeit with moderate yield (34%). Our method was then applied on substrates bearing a single methyl group, which exhibited the desired reactivity, giving the corresponding lactones in 47% (3t
1), implying that the reaction is sensitive to the electronic properties of the aryl group at this position. Next, we evaluated a series of 3-aryl-substituted aliphatic acids featuring equidistant benzylic C–H bonds, the activation of which would trigger side reactions/decomposition pathways, rendering these substrates particularly challenging. Notably, besides the simple phenyl group (3n, 40%), a range of synthetically useful functional groups, including an ester (3o, 41%), a nitro (3p, 43%), a trifluoromethylthio (3q, 44%), and a trifluoromethyl (3r, 45%) group, were found to be amenable with our protocol. Furthermore, phenyl acetic acid-derived 1s, in which ortho C(sp2)–H activation/functionalization competes with the desired reactivity,18 afforded 3s, albeit with moderate yield (34%). Our method was then applied on substrates bearing a single methyl group, which exhibited the desired reactivity, giving the corresponding lactones in 47% (3t![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3t′ = 11
3t′ = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and 50% (3u) yield, respectively. Additionally, substrate 1v, bearing a single methyl group and featuring reactive benzylic C(sp3)–H bonds, afforded the desired lactone in satisfactory yield (3v, 40%). Finally, we tested our method on gemfibrozil, an oral lipid-lowering drug, and obtained 3w in 53% yield. These results underscore the applicability of our protocol across a wide range of aliphatic carboxylic acid substrates. Next, we proceeded to study the scope with respect to the olefinic reaction partner. Styrenes with halogens at ortho (3x, 52% and 3y, 52%), meta (3z, 52% and 3aa, 48%), and para positions [3ab, 54%; 52% (3ac
1) and 50% (3u) yield, respectively. Additionally, substrate 1v, bearing a single methyl group and featuring reactive benzylic C(sp3)–H bonds, afforded the desired lactone in satisfactory yield (3v, 40%). Finally, we tested our method on gemfibrozil, an oral lipid-lowering drug, and obtained 3w in 53% yield. These results underscore the applicability of our protocol across a wide range of aliphatic carboxylic acid substrates. Next, we proceeded to study the scope with respect to the olefinic reaction partner. Styrenes with halogens at ortho (3x, 52% and 3y, 52%), meta (3z, 52% and 3aa, 48%), and para positions [3ab, 54%; 52% (3ac![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3ac′ = 12
3ac′ = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); and 3ad, 32%] underwent smooth product formation. A series of styrenes featuring various substituents at the para position was examined next. Notably, a broad range of synthetically useful functional groups, such as trifluoromethyl (61%, 3ae
1); and 3ad, 32%] underwent smooth product formation. A series of styrenes featuring various substituents at the para position was examined next. Notably, a broad range of synthetically useful functional groups, such as trifluoromethyl (61%, 3ae![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3ae′ = 3
3ae′ = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), ester (62%, 3af 47% + 3af′ 15%), nitro (61%, 3ag 51% + 3ag′ 10%), and protected amine (57%, 3ah
1), ester (62%, 3af 47% + 3af′ 15%), nitro (61%, 3ag 51% + 3ag′ 10%), and protected amine (57%, 3ah![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3ah′ = 4
3ah′ = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were found to be well tolerated, affording their respective lactones in moderate to good yields. Additionally, meta-substituted styrenes, with an aldehyde (53%, 3ai
1) were found to be well tolerated, affording their respective lactones in moderate to good yields. Additionally, meta-substituted styrenes, with an aldehyde (53%, 3ai![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3ai′ = 1.6
3ai′ = 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) or protected hydroxyl (3aj, 43%) group, as well as an ortho-substituted styrene (3ak, 52%), were found to be compatible with our protocol. Notably, since an excess of styrene is employed in our reaction, the presence of Lewis basic heteroatom-containing functional groups on the styrene could, in principle, be detrimental to the reaction outcome by acting as catalyst poisons. The successful application of our method to such cases thus demonstrates the versatility and robustness of our method. It is worth highlighting that although lactone 3 was exclusively formed in most cases, electron-deficient styrenes led to a relatively higher ratio of 3′ to 3 (see the ESI† for more details).
1) or protected hydroxyl (3aj, 43%) group, as well as an ortho-substituted styrene (3ak, 52%), were found to be compatible with our protocol. Notably, since an excess of styrene is employed in our reaction, the presence of Lewis basic heteroatom-containing functional groups on the styrene could, in principle, be detrimental to the reaction outcome by acting as catalyst poisons. The successful application of our method to such cases thus demonstrates the versatility and robustness of our method. It is worth highlighting that although lactone 3 was exclusively formed in most cases, electron-deficient styrenes led to a relatively higher ratio of 3′ to 3 (see the ESI† for more details).
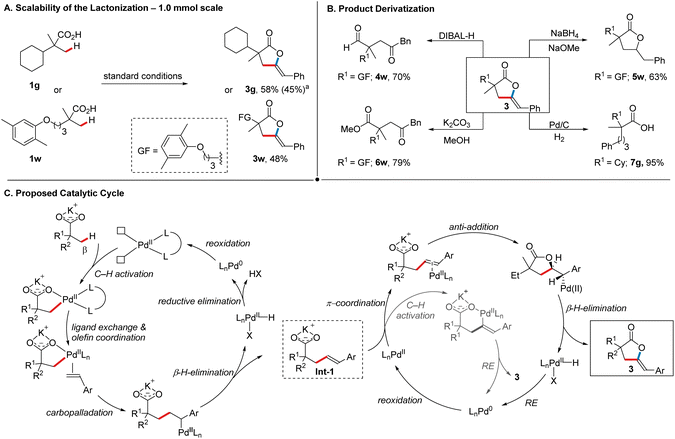
In order to further showcase the synthetic utility of our protocol, we began by testing the scalability of the process. Two representative products, 3g (58%) and 3w (48%) could be obtained in synthetically useful yields on a 1.0 mmol scale (Scheme 4A). Furthermore, scaling up the reaction to 5.0 mmol delivered 3g (45%) with a slightly reduced yet satisfactory yield, even without extensive re-optimization of the reaction conditions. Next, we investigated possible derivatization reactions of the γ-alkylidene lactones. Given the importance of 1,4-dicarbonyl motifs across various fields and the challenges associated with their synthesis,19 we evaluated the utility of γ-alkylidene lactones in this context. First, the reduction of gemfibrozil-derived lactone 3w with DIBAL-H delivered 1,4-keto aldehyde 4w in good yield (70%, Scheme 4B), while methanolysis of 3w delivered 1,4-keto ester 6w (79%). Next, selective reduction of the exocyclic double bond in 3w using NaBH4 in the presence of catalytic NaOMe afforded 5w (63%), thus offering another avenue for the synthesis of bio-active lactones.20 The treatment of 3g with 1 atm H2 in the presence of Pd/C resulted in the formation of 7g (95%). Compounds such as 7g could, for example, be further derivatized into benzosuberone analogues via an intramolecular Friedel–Crafts acylation, highlighting the potential of our method in the generation of compound libraries.21
Based on literature precedence,7b,c,22 we propose that our transformation proceeds via a PdII/Pd0 catalytic cycle, as depicted in Scheme 4C. First, the coordination of an external ligand to Pd(OAc)2 generates the active catalyst. The subsequent β-C(sp3)–H activation step is accelerated by both the counter-cation (K+) and the ligand, occurring through a CMD mechanism.22a Following this, a sequence of ligand exchange, carbopalladation, β-hydride elimination, and decoordination results in the formation of C–H olefination product Int-1.7b,c,22b The product decoordination concomitantly gives a PdII–hydride species that can undergo a reductive elimination (RE) giving a Pd0 species, which is then re-oxidized by the terminal oxidants employed in the reaction. From this stage, there are two plausible pathways that lead to the product formation: Int-1 could undergo a sequence of oxypalladation followed by β-H elimination to form 3. In this sequence, anti-oxypalladation would be favored over a syn-pathway, as indicated by the observed Z stereochemistry in 3.22b,c Alternatively, Int-1 could undergo a carboxylic acid-directed vinylic C(sp2)–H activation7e,23 and subsequent C–O RE to furnish 3. Both of these pathways result in the concomitant formation of Pd0, which is subsequently re-oxidized. Given that the olefination and cyclization steps collectively require the removal of four electrons from Pd by oxidant(s), either four equivalents of Ag(I) or a combination of silver salt, MnO2, and air are required, with the latter demonstrating superior efficiency in our case. Based on the mechanistic considerations regarding the cyclization step, we conducted a series of experiments. When independently synthesized Int-1 was subjected to the standard reaction conditions, 3 was obtained in 38% NMR-yield (see the ESI† for more details). The Z-isomer of Int-1, in contrast, gave E-configured 3 in 48% NMR-yield when subjected to the standard reaction conditions. These results indicate that Int-1 is an intermediate in our reaction and confirm a stereospecific pathway from Int-1 to the final product. Based on the current data and literature reports, neither of the proposed pathways for the final cyclization step can be fully excluded. However, considering the relative ease with which such an olefinated intermediate is expected to undergo palladium-mediated intramolecular attack by a nucleophilic functional group to form a kinetically favored five-membered ring22c,d and the relatively unfavorable formation of a thermodynamically labile six-membered palladacycle via C–H activation,24 the oxypalladation/β-H elimination pathway appears to be the more plausible scenario (for a discussion of possible pathways leading to the formation of side product 3′; see the ESI†).
Conclusions
In summary, we have developed a Pd-catalyzed triple C–H functionalization protocol for the synthesis of γ-alkylidene lactones by molecular stitching of readily available carboxylic acids and styrenes. The reaction is enabled by the use of an N-acyl sulfonamide (NASA)-ligand and exhibits excellent functional group tolerance, allowing for the efficient one-step synthesis of structurally diverse γ-alkylidene lactones. Our study demonstrates the scalability of the protocol, a number of synthetically valuable derivatizations of the obtained products, and provides mechanistic insights. This novel access to γ-alkylidene lactones is expected to prove highly useful in various disciplines. Additionally, the superior performance of N-acyl sulfonamide (NASA-) ligands in this transformation is expected to trigger further research toward the utilization of this promising ligand class.Data availability
Full experimental results are available in the ESI.†Author contributions
EC and SM: methodology, investigation, writing – original draft, review & editing. MvG: conceptualization, writing – original draft, review & editing, supervision, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge generous financial support by the DFG (Emmy Noether Programme) and Kiel University. We thank all members of our MS and NMR department for their excellent service. Furthermore, M. Luft is acknowledged for measuring IR spectra.References
- (a) E.-i. Negishi and M. Kotora, Tetrahedron, 1997, 53, 6707–6738 CrossRef CAS; (b) R. A. Amos and J. A. Katzenellenbogen, J. Org. Chem., 1978, 43, 560–564 CrossRef CAS; (c) M. Yao, W. Yang, J. Li, C. Huang, J. Fang, S. Qin, S. Liu and X. Yang, Chin. J. Chem., 2024, 42, 1509–1514 CrossRef CAS; (d) C.-H. Chen, W.-L. Lo, Y.-C. Liu and C.-Y. Chen, J. Nat. Prod., 2006, 69, 927–933 CrossRef CAS PubMed.
- (a) E.-S. J. Lee and F. K. Gleason, Plant Sci., 1994, 103, 155–160 CrossRef CAS; (b) J.-P. Surivet and J.-M. Vatèle, Tetrahedron Lett., 1996, 37, 4373–4376 CrossRef CAS; (c) S. F. Hinkley, J. A. Moore, J. Squillari, H. Tak, R. Oleszewski, E. P. Mazzola and B. B. Jarvis, Magn. Reson. Chem., 2003, 41, 337–343 CrossRef CAS; (d) W. Jia, S. Li, M. Yu, W. Chen and N. Jiao, Tetrahedron Lett., 2009, 50, 5406–5408 CrossRef CAS; (e) G. D. Smith and N. Thanh Doan, J. Appl. Phycol., 1999, 11, 337–344 CrossRef CAS.
- (a) Y. Ge, Z. Han, Z. Wang, C.-G. Feng, Q. Zhao, G.-Q. Lin and K. Ding, Angew. Chem., Int. Ed., 2018, 57, 13140–13144 CrossRef CAS PubMed; (b) T. Ohta, T. Miyake, N. Seido, H. Kumobayashi, S. Akutagawa and H. Takaya, Tetrahedron Lett., 1992, 33, 635–638 CrossRef CAS; (c) T. Ohta, T. Miyake, N. Seido, H. Kumobayashi and H. Takaya, J. Org. Chem., 1995, 60, 357–363 CrossRef CAS.
- (a) M. Sayantika Bhakta and T. Ghosh, Asian J. Org. Chem., 2021, 10, 496–505 CrossRef CAS; (b) C. Pimpasri, X. Luo, S. Yang and S. Díez-González, Adv. Synth. Catal., 2024, 366, 806–812 CrossRef CAS; (c) Q.-A. Huang, T. Ikeda, K. Haruguchi, S. Kawai, E. Yamamoto, H. Murayama, T. Ishida, T. Honma and M. Tokunaga, Appl. Catal., A, 2022, 643, 118765 CrossRef CAS; (d) F. Alonso, I. P. Beletskaya and M. Yus, Chem. Rev., 2004, 104, 3079–3160 CrossRef CAS PubMed; (e) A. Nagendiran, O. Verho, C. Haller, E. V. Johnston and J.-E. Bäckvall, J. Org. Chem., 2014, 79, 1399–1405 CrossRef CAS PubMed.
- (a) A. Uttry and M. van Gemmeren, Synlett, 2018, 29, 1937–1943 CrossRef CAS; (b) A. Uttry and M. van Gemmeren, Synthesis, 2020, 52, 479–488 CrossRef CAS; (c) A. Das and B. Maji, Chem.–Asian J., 2021, 16, 397–408 CrossRef CAS PubMed.
- (a) M. Tomanik and J.-Q. Yu, J. Am. Chem. Soc., 2023, 145, 17919–17925 CrossRef CAS PubMed; (b) H. Park and J.-Q. Yu, J. Am. Chem. Soc., 2020, 142, 16552–16556 CrossRef CAS PubMed.
- (a) Z. Zhuang, C.-B. Yu, G. Chen, Q.-F. Wu, Y. Hsiao, C. L. Joe, J. X. Qiao, M. A. Poss and J.-Q. Yu, J. Am. Chem. Soc., 2018, 140, 10363–10367 CrossRef CAS PubMed; (b) K. K. Ghosh, A. Uttry, A. Mondal, F. Ghiringhelli, P. Wedi and M. van Gemmeren, Angew. Chem., Int. Ed., 2020, 59, 12848–12852 CrossRef CAS PubMed; (c) J. Das, P. Dolui, W. Ali, J. P. Biswas, H. B. Chandrashekar, G. Prakash and D. Maiti, Chem. Sci., 2020, 11, 9697–9702 RSC; (d) H. S. Park, Z. Fan, R.-Y. Zhu and J.-Q. Yu, Angew. Chem., Int. Ed., 2020, 59, 12853–12859 CrossRef CAS PubMed; (e) T. Sheng, Z. Zhuang, Z. Wang, L. Hu, A. N. Herron, J. X. Qiao and J.-Q. Yu, J. Am. Chem. Soc., 2022, 144, 12924–12933 CrossRef CAS PubMed; (f) G. Naskar and M. Jeganmohan, Org. Lett., 2024, 26, 6580–6585 CrossRef CAS PubMed.
- H. Jiang, J. He, T. Liu and J.-Q. Yu, J. Am. Chem. Soc., 2016, 138, 2055–2059 CrossRef CAS PubMed.
- (a) Y. Ding, Y.-Q. Han, L.-S. Wu, T. Zhou, Q.-J. Yao, Y.-L. Feng, Y. Li, K.-X. Kong and B.-F. Shi, Angew. Chem., Int. Ed., 2020, 59, 14060–14064 CrossRef CAS PubMed; (b) L.-S. Wu, Y. Ding, Y.-Q. Han and B.-F. Shi, Org. Lett., 2021, 23, 2048–2051 CrossRef CAS PubMed; (c) L.-S. Wu, T. Zhou and B.-F. Shi, Org. Lett., 2024, 26, 4457–4462 CrossRef CAS PubMed.
- (a) S. Mal, F. Jurk, K. Hiesinger and M. van Gemmeren, Nat. Synth., 2024, 3, 1292–1298 CrossRef CAS; (b) T. Xu, S. Mal and M. van Gemmeren, ACS Catal., 2025, 15, 2735–2741 CrossRef CAS; (c) K. K. Ghosh, A. Uttry, A. Koldemir, M. Ong and M. van Gemmeren, Org. Lett., 2019, 21, 7154–7157 CrossRef CAS PubMed.
- K. M. Engle, D.-H. Wang and J.-Q. Yu, J. Am. Chem. Soc., 2010, 132, 14137–14151 CrossRef CAS PubMed.
- (a) P.-X. Shen, L. Hu, Q. Shao, K. Hong and J.-Q. Yu, J. Am. Chem. Soc., 2018, 140, 6545–6549 CrossRef CAS PubMed; (b) F. Ghiringhelli, A. Uttry, K. K. Ghosh and M. van Gemmeren, Angew. Chem., Int. Ed., 2020, 59, 23127–23131 CrossRef CAS PubMed.
- P. Lassalas, B. Gay, C. Lasfargeas, M. J. James, V. Tran, K. G. Vijayendran, K. R. Brunden, M. C. Kozlowski, C. J. Thomas, A. B. Smith III, D. M. Huryn and C. Ballatore, J. Med. Chem., 2016, 59, 3183–3203 CrossRef CAS PubMed.
- M. Farizyan, A. Mondal, S. Mal, F. Deufel and M. van Gemmeren, J. Am. Chem. Soc., 2021, 143, 16370–16376 CrossRef CAS PubMed.
- D. A. Strassfeld, C.-Y. Chen, H. S. Park, D. Q. Phan and J.-Q. Yu, Nature, 2023, 622, 80–86 CrossRef CAS PubMed.
- (a) M. Janssen and D. E. De Vos, Chem.–Eur. J., 2019, 25, 10724–10734 CrossRef CAS PubMed; (b) H. S. S. Chan, J.-M. Yang and J.-Q. Yu, Science, 2022, 376, 1481–1487 CrossRef CAS PubMed.
- J. Das, W. Ali, A. Ghosh, T. Pal, A. Mandal, C. Teja, S. Dutta, R. Pothikumar, H. Ge, X. Zhang and D. Maiti, Nat. Chem., 2023, 15, 1626–1635 CrossRef CAS PubMed.
- D.-H. Wang, T.-S. Mei and J.-Q. Yu, J. Am. Chem. Soc., 2008, 130, 17676–17677 CrossRef CAS PubMed.
- (a) G. Piancatelli, M. D'Auria and F. D'Onofrio, Synthesis, 1994, 1994, 867–889 CrossRef; (b) S. Mondal, N. Chatterjee and S. Maity, Chem.–Eur. J., 2023, 29, e202301147 CrossRef CAS PubMed; (c) M. Lemmerer, M. Schupp, D. Kaiser and N. Maulide, Nat. Synth., 2022, 1, 923–935 CrossRef CAS.
- (a) V. U. Pawar, S. Ghosh, B. A. Chopade and V. S. Shinde, Bioorg. Med. Chem. Lett., 2010, 20, 7243–7245 CrossRef CAS PubMed; (b) Y. Usuki, Y. Tanaka, M. Morii and T. Satoh, Org. Biomol. Chem., 2023, 21, 2398–2404 RSC.
- (a) R. P. Tanpure, C. S. George, T. E. Strecker, L. Devkota, J. K. Tidmore, C.-M. Lin, C. A. Herdman, M. T. MacDonough, M. Sriram, D. J. Chaplin, M. L. Trawick and K. G. Pinney, Bioorg. Med. Chem., 2013, 21, 8019–8032 CrossRef CAS PubMed; (b) B. Yadagiri, U. D. Holagunda, R. Bantu, L. Nagarapu, C. G. Kumar, S. Pombala and B. Sridhar, Eur. J. Med. Chem., 2014, 79, 260–265 CrossRef CAS PubMed; (c) Y. Tian, M. A. Shehata, S. J. Gauger, C. Veronesi, L. Hamborg, L. Thiesen, J. Bruus-Jensen, J. S. Royssen, U. Leurs, A. S. G. Larsen, J. Krall, S. M. Ø. Solbak, P. Wellendorph and B. Frølund, J. Med. Chem., 2022, 65, 15066–15084 CrossRef CAS PubMed.
- (a) K. M. Engle, Pure Appl. Chem., 2016, 88, 119–138 CrossRef CAS; (b) Y. Lu, D.-H. Wang, K. M. Engle and J.-Q. Yu, J. Am. Chem. Soc., 2010, 132, 5916–5921 CrossRef CAS PubMed; (c) R. M. Trend, Y. K. Ramtohul and B. M. Stoltz, J. Am. Chem. Soc., 2005, 127, 17778–17788 CrossRef CAS PubMed; (d) P. Kočovský and J.-E. Bäckvall, Chem.–Eur. J., 2015, 21, 36–56 CrossRef PubMed.
- (a) R. Giri and J.-Q. Yu, J. Am. Chem. Soc., 2008, 130, 14082–14083 CrossRef CAS PubMed; (b) B. Ramesh and M. Jeganmohan, J. Org. Chem., 2022, 87, 5668–5681 CrossRef CAS PubMed; (c) S. Yu, C. Hong, Z. Liu and Y. Zhang, Org. Lett., 2021, 23, 8359–8364 CrossRef CAS PubMed; (d) Y. Jiang, P. Li, J. Zhao, B. Liu and X. Li, Org. Lett., 2020, 22, 7475–7479 CrossRef CAS PubMed; (e) Y. Gao, J. Nie, Y. Li, X. Li, Q. Chen, Y. Huo and X.-Q. Hu, Org. Lett., 2020, 22, 2600–2605 CrossRef CAS PubMed.
- P. Dolui, J. Das, H. B. Chandrashekar, S. S. Anjana and D. Maiti, Angew. Chem., Int. Ed., 2019, 58, 13773–13777 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sc03349g |
| ‡ Equal contribution. |
| This journal is © The Royal Society of Chemistry 2025 |