 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
How do you (dis)solve a problem like methylene chloride?†
James Sherwood
Green Chemistry Centre of Excellence, University of York, Heslington, YO10 5DD, UK. E-mail: james.sherwood@york.ac.uk
First published on 15th July 2025
Abstract
Chemical regulation in the European Union (EU) and the United States of America (USA) has restricted the use of some historically important solvents, essentially banning certain uses. The most high profile regulatory action thus far has been a ‘Final Risk Management Rule’ prohibiting all consumer uses, and many commercial uses, of methylene chloride (dichloromethane, DCM) by the United States Environmental Protection Agency (EPA) through the Toxic Substances Control Act (TSCA). The unique properties of chlorinated solvents makes direct substitution difficult or impossible for most uses, and creative solutions are needed. The replacement of methylene chloride in synthesis, extraction, and chromatography with green solvents will be discussed as a way of using regulatory intervention as the catalyst for innovation and positive change.
Sustainability spotlightNew restrictions on how methylene chloride can be used means that there is a need for alternative solvents, and a danger that the replacements introduce new risks and unforeseen consequences. This work establishes how and where methylene chloride is being used at present, and what solvents offer a viable alternative. Solvent substitution in favour of less hazardous solvents contributes to the aspirations of Good Health and Well-being (SDG3) and Climate Action (SDG13). Chemical processes that utilise safer solvents to comply with regulation promote Industry, Innovation and Infrastructure (SDG9) and permit Responsible Consumption and Production (SDG12). |
Introduction
Methylene chloride, also known as dichloromethane (DCM), is one of the most useful and popular organic solvents. Methylene chloride was discovered in 1840 by Henri Victor Regnault when they chlorinated methyl chloride. Commercial production of methylene chloride was first performed by the reaction of chloroform with zinc and hydrochloric acid.1 This was replaced by the chlorination of methane at 500 °C which results in a mixture of chloromethanes (methyl chloride, methylene chloride, chloroform, carbon tetrachloride). As methylene chloride gained popularity in the mid twentieth century, earlier manufacturing techniques were surpassed by the contemporary method of producing methyl chloride from methanol and hydrogen chloride, which is subsequently chlorinated (Fig. 1).2 The main uses of methylene chloride which established it as a mainstay solvent were as a paint remover, for decaffeination, the production of polycarbonates, and as a blowing agent for polyurethane and the subsequent cleaning of machinery.2 Over time, methylene chloride was replaced in some of these applications due to health concerns, a trend culminating in recent regulatory action.We can gain some insight into modern methylene chloride use in the USA from the Environmental Protection Agency (EPA) Toxics Release Inventory (data for 2020 to 2023 is shown in Fig. 2).3 The data up to 2022 shows emissions of methylene chloride topping 1 million litres each year. The 2023 data set records 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 litres of methylene chloride emissions, a notable drop, which could indicate the start of a concerted effort to reduce methylene chloride use. The industrial sector responsible for the most methylene chloride emissions is chemical manufacturing. Cleaning machinery is also a source of methylene chloride emissions. Some emissions are attributed to hazardous waste processors (i.e. off site emissions). Each year, the vast majority of methylene chloride emissions are to the air, either as intentional emissions or as fugitive emissions.
000 litres of methylene chloride emissions, a notable drop, which could indicate the start of a concerted effort to reduce methylene chloride use. The industrial sector responsible for the most methylene chloride emissions is chemical manufacturing. Cleaning machinery is also a source of methylene chloride emissions. Some emissions are attributed to hazardous waste processors (i.e. off site emissions). Each year, the vast majority of methylene chloride emissions are to the air, either as intentional emissions or as fugitive emissions.
The reasons why chlorinated solvents are highly valued have been covered elsewhere.4 In brief, methylene chloride has the benefits of halogen bonding,5 providing strong interactions with typical dipolar substrates for high solubility, while at the same time having a low boiling point (40 °C) for easy removal. This combination of properties is extremely convenient for laboratory and manufacturing uses, but as explained next, comes at the cost of human health and environmental damage.
Hazards and regulatory action
Since April 2024, the United States Environmental Protection Agency (EPA) has issued an intervention via the Toxic Substances Control Act (TSCA) to reduce the risk to human health posed by methylene chloride.6 It was found that methylene chloride poses an unacceptable risk of cancer,7 acute neurotoxicity, and chronic liver toxicity due to exposure arising from many of its uses.8 The final risk management rule affects methylene chloride in the USA in the following ways.9 All consumer uses of methylene chloride have been banned since May 2025. The following uses of methylene chloride are permitted within industrial and commercial operations, as well as research, government, and academic institutions, but a ‘Workplace Chemical Protection Program’ (WCPP) is required: manufacturing of methylene chloride, import of methylene chloride, uses of methylene chloride as a reactant, incorporation into a mixture (e.g. formulation), repackaging (e.g. rebottling) of methylene chloride containing products, recycling of methylene chloride, its use as a laboratory chemical (i.e. for research and teaching purposes), as a paint or coating remover for aircraft and spacecraft, as a bonding agent for solvent welding, as a ‘processing aid’ – the example given in the legislation is of a heat transfer fluid but it can also be understood to include solvent uses,9 in plastics and rubber manufacturing, in closed loop processes where the methylene chloride is reclaimed, and methylene chloride disposal (waste management). A WCPP consists of monitoring the airborne methylene chloride concentration and compliance with exposure limits, as well as requirements for protective equipment and training and an exposure control plan.10 Furthermore, the 8 hours time-weighted average exposure limit has been lowered from 25 to 2 ppm, and the short-term exposure limit has been lowered from 125 to 16 ppm.11 Commercial uses of methylene chloride not previously listed or able to comply to a WCPP are banned after April 2026. There are some important exceptions where the TSCA ruling does not apply, including the pharmaceutical industry (accounting for about a third of methylene chloride use) and food processing (e.g. decaffeination) which are covered by other legislation.The number of complete TSCA final risk management rules is now five, also covering carbon tetrachloride, perchloroethylene, trichloroethylene, and asbestos.12 It is not a coincidence that four of the five finalised risk management rules target hazardous chlorinated solvents. Risk management rules are in the pipeline for a variety of other solvents, including 1,2-dichloroethane, which is on the REACH authorisation list in Europe.13 Methylene chloride is also subject to EU restrictions, specifically forbidding its use in paint removers.14
The health hazards, environmental impact, and safety (physical) hazards of solvents can be ranked using the Safe and Sustainable by Design (SSbD) approach pioneered in the EU (Fig. 3).15 Compared to other chlorinated solvents, the hazards of methylene chloride are less severe, which contributed to its status as a safer option compared to chloroform or 1,2-dichloroethane for example in early solvent selection guides.16 This option is no longer tenable for applications not permitted under the recent TSCA risk evaluation of course. Methylene chloride is suspected of causing cancer, as designated with the hazard statement H351. Methylene chloride is also an irritant. 1,2-Dichloroethane and trichloroethylene definitively cause cancer (H350) and are regarded as more hazardous in this respect. The chronic organ damage (H372) of chloroform and carbon tetrachloride is also highly concerning and warrants a greater health risk score than methylene chloride in the SSbD framework. Regarding physical hazards, methylene chloride is not flammable and generally stable, and has no formal hazard statements regarding environmental toxicity. Fig. 3 also includes potential replacements for methylene chloride, all of which have lower health risks but tend to be flammable or potentially explosive in the case of ethers. The CHEM21 solvent selection guide ranks ethers poorly due to these safety concerns.17 The transition from non-flammable chlorinated solvents to flammable hydrocarbon and oxygenated solvents will require adjustments to safety procedures.
 | ||
| Fig. 3 Radar charts: hazard severity levels in health, environment and physical hazard categories as defined by the EU safe and sustainable by design guidelines. Hazard severity is categorised in four levels, from inside to outside on each radar chart: no applicable hazards, minor hazards, concerning hazards, most severe hazards. Blocks: underneath each radar plot are the greenness scores from the CHEM21 solvent selection guide, in order of safety, health, and environment. Hazard codes and further information are given in the ESI.† | ||
Despite the inherent hazards of methylene chloride being less severe than other chlorinated solvents, ultimately the risk of using methylene chloride also depends on exposure. Chronic toxicity (e.g. cancer) requires repeated or prolonged exposure to become a relevant mode of action. That said, there is a lot of evidence for the acute toxicity of methylene chloride, including death, that ultimately resulted in the regulatory action being taken. Since 1980, 85 deaths have occurred directly due to the use of methylene chloride in the USA.18 Seventy four of those fatalities occurred at work, with the use of paint strippers in enclosed spaces the main culprit.19 Exposure to methylene chloride vapor causes heart attacks, which can occur after only brief accidental exposure, for example from leaks.20 There have also been reports of necrosis after accidental injection of methylene chloride in laboratory accidents.21 Less typically, physical risks can occur from the use of methylene chloride. The combination of methylene chloride and sodium azide can form the explosive diazidomethane.22 It is generally best practice to avoid using halogenated solvents with azides for this reason.
Chlorinated solvents are known to deplete stratospheric ozone, the most notorious of which is carbon tetrachloride, earning itself a place amongst the substances banned under the conditions of the Montreal Protocol.23 Methylene chloride also depletes ozone,24 and worldwide emissions have been recorded as increasing,25 posing a greater threat to the environment. Therefore, evidence suggests methylene chloride poses greater risks to people and the planet than might be expected from hazard statements alone.
Uses of methylene chloride
Formulation
It is perhaps paint remover products that have been the most high profile use of methylene chloride and the main driver behind legislative action. Use of paint stripper products containing methylene chloride was banned in the EU in 2012,26 and banned for consumers in the USA in 2019 (prior to the more recent ruling introducing wider restrictions on methylene chloride use).27Alternative solvents have been suggested to reformulate paint removers. A mixture of methanol and DMSO has been suggested, primarily on the basis of environmental impact rather than technical performance.28 Milescu et al. tested dihydrolevoglucosenone (brand name Cyrene) for its ability to remove paint from porous substrates such as brick with some success compared to other neat solvents.29 However, neither of these solutions is a formulated product. Contemporary, commercially available paint removal products contain a variety of ingredients, often alkanes, alkoxy alcohols, and a base (hydroxide or amine, see Table S2 of the ESI†). These products are corrosive but do not present a chronic toxicity hazard because of the absence of methylene chloride.
Synthetic chemistry
Although there is concern over the future of methylene chloride in industrial chemistry, in those research labs shielded from the brunt of regulations, the use of methylene chloride has not wavered. A survey of some representative journals shows that reported reactions in methylene chloride are just as popular as they were in 2020 (Fig. 4). ACS Sustainable Chemistry and Engineering has the lowest instances of methylene chloride being used as a solvent at just 1 reported reaction in every 10 original research articles. RSC Sustainability in 2023 also had an average of 0.1 reactions using methylene chloride as a solvent per article. Green Chemistry reports a reaction in methylene chloride an average of 1–2 times per article. Journals more likely to contain substrate screenings of a synthetic methodology or catalogues of novel compounds have more instances of methylene chloride use per article, and likely much more is used in extractions and chromatography. A prime example is the Journal of Medicinal Chemistry, featuring an average of 12 reactions in methylene chloride per article.Taking a more detailed look at articles published in Organic Process Research and Development (OPRD) between January and March 2025, which regularly covers the kilogram synthesis of fine chemicals, the choice of solvents for reactions, work up (extraction or trituration or solvent swap) and recrystallisation, and column chromatography was surveyed. Methylene chloride accounts for 10–15% of reported solvent use and it was the third most common choice of solvent in each category (if alkanes are grouped together). Methanol and tetrahydrofuran (THF) are the most frequently used options for reaction solvents (closely followed by methylene chloride), while ethyl acetate and alkanes (with n-heptane the most popular) are the most common post-reaction solvents (again, methylene chloride is third). A previous survey of OPRD covering the years 1997 to 2012 found between 17% and 64% of the papers examined featured methylene chloride with a mean annual occurrence of 38%.30 The equivalent figure for 2025 (first quarter) is 32%. Overall, the use of methylene chloride appears to fluctuate without a strong trend but it is always significant. We can infer the popularity of methylene chloride in small scale exploratory reactions is still transferring onto larger scale and pilot plant chemistry.
Chromatography in safer solvents
When utilised, flash chromatography is the greatest source of waste in a chemical process, and the quantity of solvent required is the main contributor. Ethyl acetate combined with alkanes is a common option, but methanol–methylene chloride is considered superior for the separation of polar substances. A visual guide for greener chromatography, showing proportions of alternative solvents has been designed by Taygerly et al. (Fig. 5).31 Heptane or methyl tert-butyl ether (MTBE) modified with 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethyl acetate-ethanol is a broadly useful alternative. Note that MTBE scores poorly in the CHEM21 solvent selection guide due to its flammability and resistivity.17 This presents a new challenge because methylene chloride is non-flammable and so risk assessments must account for new safety hazards. All the recommended solvent systems contain flammable solvents and pose some health hazards. The practitioner must be aware of these hazards, especially because purification solvents are required in greater quantities than reaction solvents (and must not be overlooked in risk assessments). Another seminal study by MacMilan et al. concluded that cyclopentyl methyl ether (CPME)-methanol has the potential to replace methylene chloride-methanol in chromatography. To avoid toxic methanol, an isopropanol (IPA)-ethyl acetate eluent can also be used.32 Heptane in combination with methyl acetate or ethyl acetate has also been supported for the separation of analgesics.33
1 ethyl acetate-ethanol is a broadly useful alternative. Note that MTBE scores poorly in the CHEM21 solvent selection guide due to its flammability and resistivity.17 This presents a new challenge because methylene chloride is non-flammable and so risk assessments must account for new safety hazards. All the recommended solvent systems contain flammable solvents and pose some health hazards. The practitioner must be aware of these hazards, especially because purification solvents are required in greater quantities than reaction solvents (and must not be overlooked in risk assessments). Another seminal study by MacMilan et al. concluded that cyclopentyl methyl ether (CPME)-methanol has the potential to replace methylene chloride-methanol in chromatography. To avoid toxic methanol, an isopropanol (IPA)-ethyl acetate eluent can also be used.32 Heptane in combination with methyl acetate or ethyl acetate has also been supported for the separation of analgesics.33
The chromatographic separation of acetaminophen and caffeine is a common system to study new solvents. Interpreting thin layer chromatography (TLC) retardation factors (Rf) with a statistical thermodynamic approach to intermolecular interactions has suggested methyl pivalate, tert-butyl acetate, sec-butyl acetate and ethyl isobutyrate are suitable replacements for methylene chloride in combination with methanol.34 Similarly, Sharma et al. used TLC to identify methyl acetate and ethyl acetate as replacements for methylene chloride.35 Instead of adapting conventional preparatory chromatography, alternative purification techniques can be adapted. Peterson et al. suggest investigating ion exchange resins, reverse-phase chromatography, or supercritical fluid chromatography.36
Replacing methylene chloride in extractions
Aside from column chromatography, the greatest use of methylene chloride within a synthetic process will often be the work up. This is where water soluble by-products and other contamination is separated from a solution of the primary product. Other types of extractions are also used in the analysis of environmental samples, restoration, and food manufacturing (e.g. oil extraction or decaffeination). Ethyl acetate is also a common extraction solvent and sometimes interchangeable with methylene chloride. For instance, lake sediment extraction for environmental monitoring purposes has been achieved with ethyl acetate, albeit with some loss of recovery, particularly of fatty acids, compared to methylene chloride.37 There are more reasons why methylene chloride might be preferred over ethyl acetate or other alternatives. The solubility of water in methylene chloride is an order of magnitude lower than water in ethyl acetate,38 limiting cross contamination and the possibility of emulsions forming. The high density of methylene chloride is advantageous when removing it from water by separating funnel and this is particularly true at larger manufacturing scales. Ethyl acetate is also flammable, whereas methylene chloride is not. Methylene chloride has a lower boiling point for easier removal too, although this can translate to more fugitive emissions.Ideally, the need for extraction solvents should be completely eliminated. In one innovation, glass powder coated with an absorbent silicone polymer is added to water to remove organic compounds, which then can be filtered and added as the input for column chromatography.39 Obviously the high solvent demand of the column is not solved, but significant solvent savings were reported all the same. Similarly, a polyurethane foam sponge does the same job in place of a traditional solvent extraction.40
Where a solvent is still required, Cayot et al. used an acetone-cyclopentane or ethyl acetate-ethanol mixture for food extractions in the food processing industry.41 Caffeine extraction is the most well known example of where methylene chloride has traditionally been used in the food industry, for which several alternative solvents are known, including ethyl lactate,42 and supercritical carbon dioxide.43 Note there are regulations in the USA and elsewhere controlling the use of solvents for food applications. Rather than banning solvents, regulation for the food sector tends to describe which solvents you can use from restrictive lists. Methylene chloride is in fact permitted for the decaffeination of coffee if the solvent residue is present in less than 10 ppm as described in USA regulation,44 or 2 ppm in the equivalent EU law.45 This approach to regulation does not encourage innovation, because novel, safer solvents suitable for food extraction will not be permitted without revisions to the law.
Methylene chloride-free synthesis
Much of the published solvent substitution activities are focused on organic synthesis, especially within the pharmaceutical industry.46 The EPA risk management rule for methylene chloride does not extend to the pharmaceutical sector, which is outside the jurisdiction of TSCA, and is an industry that routinely handles very hazardous substances with high safety standards (thereby minimising risk to acceptable levels). Nevertheless, the impressive efforts made by the pharmaceutical industry to reduce methylene chloride use shows regulation is not always required as a driver for greener practices.47–49Beyond the pharmaceutical sector, it is valuable to know what uses of methylene chloride will be impacted most by regulation. Of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 627
627![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 689 uses of methylene chloride recorded in the SciFinder database between 2020–2025 (as of March 2025), 8 reaction categories stand out as the most important (Fig. 6). Almost a quarter of the recently recorded uses of methylene chloride can be attributed to amide formation, usually between a carboxylic acid and an amine using a coupling agent (distribution of methylene chloride reactions is shown on the x-axis of Fig. 7). The equivalent process of making esters with alcohols is the third most prevalent reaction type. The hydrolysis of amides (ranked 2nd) and esters (8th) are also common reactions, specifically the cleavage of the tert-butyloxycarbonyl (boc) protecting group and tert-butyl esters respectively with trifluoroacetic acid (TFA). The prominence of amide formation and boc deprotection as methylene chloride uses are indicative of the essential role of this chemistry in contemporary bioactive molecule research, where large numbers of small molecule amides and peptides are produced for activity screening.
689 uses of methylene chloride recorded in the SciFinder database between 2020–2025 (as of March 2025), 8 reaction categories stand out as the most important (Fig. 6). Almost a quarter of the recently recorded uses of methylene chloride can be attributed to amide formation, usually between a carboxylic acid and an amine using a coupling agent (distribution of methylene chloride reactions is shown on the x-axis of Fig. 7). The equivalent process of making esters with alcohols is the third most prevalent reaction type. The hydrolysis of amides (ranked 2nd) and esters (8th) are also common reactions, specifically the cleavage of the tert-butyloxycarbonyl (boc) protecting group and tert-butyl esters respectively with trifluoroacetic acid (TFA). The prominence of amide formation and boc deprotection as methylene chloride uses are indicative of the essential role of this chemistry in contemporary bioactive molecule research, where large numbers of small molecule amides and peptides are produced for activity screening.
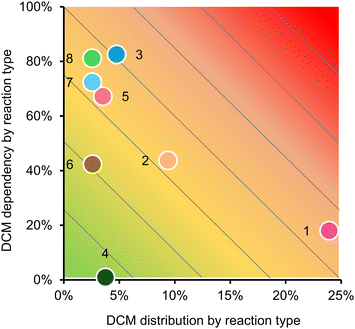 | ||
| Fig. 7 Reaction survey results represented by the prevalence of each reaction (from 1.6 million hits where methylene chloride (DCM) is the reaction solvent) on the x-axis plotted against how often DCM is used in the generic reactions of the accompanying schemes in Fig. 6 (y-axis), all according to SciFinder for the period January 2020 to March 2025. | ||
Fourth on the list of common reactions is nucleophilic substitution by amines, consisting of aromatic nucleophilic substitution (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 examples) and nucleophilic substitution (21
000 examples) and nucleophilic substitution (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 examples). The other top uses of methylene chloride as a reaction solvent are the production of sulphonamides, reductive amination, and ether cleavage. Nucleophilic addition to a carbonyl accounts for almost half the reported uses of methylene chloride as a reaction solvent. Carbonyl addition is very solvent dependent, the kinetics of which are inversely proportional to the hydrogen bond accepting ability of the solvent.50 This makes the poorly hydrogen bonding chlorinated solvents and aromatic solvents very appealing despite their hazards.
000 examples). The other top uses of methylene chloride as a reaction solvent are the production of sulphonamides, reductive amination, and ether cleavage. Nucleophilic addition to a carbonyl accounts for almost half the reported uses of methylene chloride as a reaction solvent. Carbonyl addition is very solvent dependent, the kinetics of which are inversely proportional to the hydrogen bond accepting ability of the solvent.50 This makes the poorly hydrogen bonding chlorinated solvents and aromatic solvents very appealing despite their hazards.
Fig. 7 (y-axis) also shows the dependency of the generic reaction types on methylene chloride (i.e. the percentage of reactions in each category performed in methylene chloride). This is based on the reaction schemes in Fig. 6, which represent the most common example in each reaction category. It is important to consider this, because it demonstrates that although there are many more examples of methylene chloride being used in amidations than other reactions, that reaction category is so large that actually only a minority of direct amidations using coupling agents require methylene chloride. This could indicate that the use of methylene chloride in amidations is easily eliminated by following other common protocols in other solvents. Four of the 8 reactions are predominantly performed in methylene chloride: direct esterification by coupling agent, sulphomamidations with sulphonyl chlorides, conversion of aryl methyl ethers to phenols, and TFA hydrolysis of tert-butyl esters. Aromatic nucleophilic substitution on the other hand is rarely practiced in methylene chloride and accounts for a modest amount of total methylene chloride use in synthesis. Notable solvent substitutions for the major reactions reliant on methylene chloride will now be summarised.
Amidation
Direct amide formation is necessary in a variety of fields, notably for synthetic peptides and other bioactive compounds.51 The reaction between a carboxylic acid and an amine can be achieved with a coupling agent. Analysing hexafluorophosphate azabenzotriazole tetramethyl uronium (HATU) mediated transformations,52 DMF is the most common solvent (74% of SciFinder examples since 2020), with methylene chloride accounting for the solvent choice in 18% of examples. However, because amide coupling is so prevalent in synthetic chemistry, it accounts for nearly a quarter of the reactions using methylene chloride in the SciFinder database and thus is worthy of attention.The solvent Cyrene has been used successfully in HATU mediated coupling reactions to make small molecule amides including dipeptides.53 Cyrene can also be used as a solvent in the reaction of acid chlorides and amines to make amides.54 Both reports make specific mention of washing the Cyrene out of the crude product mixture with water.53,54 Unlike methylene chloride, Cyrene is miscible with water, but needs considerable washing to completely remove.
Solid phase synthesis is a vital technology for amidation but reliant on solvents that swell the resin support. Propylene carbonate can be used as the solvent for solid phase peptide synthesis, as was demonstrated with the synthesis of nonapeptide bradykinin.55
Acetonitrile was demonstrated to give superior conversion and selectivity compared to DMF in the coupling of hindered carboxylic acids with weakly nucleophilic amines using N-acyl imidazolium intermediates in combination with tetramethylchloroformamidinium hexafluorophosphate (TCFH).56
A large screening of acid–amine pairs and coupling agents has suggested that (1-cyano-2-ethoxy-2-oxoethylidenaminooxy)dimethylamino-morpholino-carbenium hexafluorophosphate (COMU) is actually a preferable coupling agent to HATU,57 which works well in combination with dimethyl carbonate, ethyl acetate, and 2-methyltetrahydrofuran.58 Another methodology uses a dithiocarbonate to replace typical coupling agents, which can be performed in ethyl acetate.59 Other additions to the literature include the use of aqueous surfactants,60 and deep eutectic solvents.61 There are also instances of solvent-free amidation: to this end ball milling of esters and amines is known,62 as is the iodine mediated Boc protection of amines with Boc2O.63
Due to the toxicity of amide coupling agents,64 catalytic amidation methods are desirable, but have not superseded the established coupling agent protocols. These catalytic methods can be biocatalytic,65 which are possible in a variety of solvents, or chemocatalytic,66 which tend to rely on toluene and tetrahydrofuran (THF). Despite scoring slightly better in solvent selection guides than methylene chloride,17 toluene and THF both have chronic toxicity hazards.
Amide (and carbamate) hydrolysis
The deprotection of tert-butyloxycarbonyl (boc)-protected amines with trifluoroacetic acid is a major category of amide hydrolysis. 44% of recent examples are conducted in methylene chloride, and the only other popular solvent option is 1,4-dioxane (23%), which is known to cause cancer. Furthermore, trifluoroacetic acid is under scrutiny as a persistent environmental pollutant.67 Therefore, safer solvents/reagents for amide hydrolysis can be considered a priority action area despite some other reactions having a stronger dependence on methylene chloride. Fortunately there are several protocols for the thermally-initiated removal of Boc groups, reported to occur in flow reactors in acetonitrile,68 as well as in trifluoroethanol,69 or water.70,71 Instead of using trifluoroacetic acid, alternative conditions have been developed, either solvent-free with hydrogen chloride,72 or using oxalyl chloride in methanol.73Esterification
Steglich esterifications between carboxylic acids and alcohols using a carbodiimide coupling agent are usually performed in methylene chloride. Solubility and stability plays an essential role in most reactions, but additionally chlorinated solvents are especially suited to a variety of esterification methods because they accelerate the reaction through entropic effects.50,74 Despite this, a solvent selection guide documents several viable alternative solvents.75 The favoured reaction system appears to be 2-chloro-1-methylpyridinium iodide (Mukaiyama's reagent) in dimethyl carbonate. 5-Nitro-4,6-dithiocyanatopyrimidine (NDTP) can also be used as a coupling reagent in excess alcohol (as solvent and reactant), or acetonitrile as the solvent.76 1-Propanephosphonic anhydride (T3P) and cyclopentanone have been found to promote the synthesis of thioesters from thiols and carboxylic acids.77Luis et al. have demonstrated the action of TCFH upon acyl imidazolium intermediates in acetonitrile as the solvent to produce esters and thioesters.78 The method is mild (0–25 °C, ∼3 hours) and applicable to poorly reactive substrates.
Aromatic nucleophilic substitution
The typical aromatic nucleophilic substitution of fluorine with an amine tends to be performed in dimethyl sulphoxide (DMSO, 33%) or N,N-dimethylformamide (DMF, 26%) rather than chlorinated solvents. Dipolar aprotic solvents have their own issues, with DMSO prone to decompose and transfer solutes through the skin, and DMF being reprotoxic. Alternatives to dipolar aprotic solvents have been discussed elsewhere.79Sulphonamidation
Two-thirds of recently reported sulphonamidations (from the sulphonyl chloride and an amine) are performed in methylene chloride, and although THF and DMF are also popular solvents they do not represent greener alternatives on account of their respective suspected carcinogenicity and reproductive toxicity. When it concerns the use of (sulphonic or carboxylic) acid chlorides, anhydrous solvents tend to be used to avoid hydrolysis of the reactant. However, it has been shown that a pH of 8 (or lower) is sufficient to suppress hydrolysis and permit facile sulphonamidation in water.80 Alternative methods from nitroarenes instead of amines also work with water as the solvent.81 Conditions utilising deep eutectic mixtures based on choline chloride as solvents,82 or no solvent at all,83 are also known.Reductive amination
The reductive amination of aldehydes has a very strong dependence on chlorinated solvents. Where sodium triacetoxyborohydride (STAB) is the reducing agent, 42% of recent reports are in methylene chloride and a further 25% are in 1,2-dichloroethane. The problem of chlorinated solvent use in the reductive amination of aldehydes has been comprehensively addressed by the work of McGonagle et al..84 Ethyl acetate and dimethyl carbonate were found to be green, high performance alternative solvents that work on a wide range of substrates.Ether hydrolysis
The use of methylene chloride in ether hydrolysis is tied to a specific methodology for the demethylation of anisoles (methoxybenzenes) with boron trifluoride dating back to the 1960s.85 The mechanism requires the bromination of the methyl group. In the intervening years, alternative methods have been developed, such as the use of aluminium triiodide in acetonitrile,86 or an oxidative route using 2-iodoxybenzoic acid (IBX) in a mixture of dimethyl carbonate and water.87 Biocatalytic methods are also known to proceed in DMSO for guaiacol and its derivatives.88Ester hydrolysis
The hydrolysis of tert-butyl esters with TFA has an overwhelming dependence on methylene chloride (81% of recent examples). There are some examples of using water as a solvent for this transformation, including the synthesis of saccharide oligomers,89 and radiolabelled medical imaging compounds.90Other notable reactions
Naturally methylene chloride is used in many other reactions outside the top 8 summarised above. Brominations are commonly conducted in chlorinated solvents, but ethyl acetate can be an acceptable substitute.91 Olefin metathesis is another reaction overwhelmingly reliant on chlorinated solvents, but dimethyl carbonate and ethyl acetate have been shown to be suitable for ring closing metathesis reactions in particular.92 A mechanistic study was used to investigate alternative solvents for nitrene transfer reactions, which found that dimethyl carbonate, isopropyl acetate and anisole may be good candidates.93Conclusions
We are at a transitional point in the history of methylene chloride. Once viewed as a vital and irreplaceable solvent, its commercial uses are soon to be relegated to small scale exploratory chemistry, pharmaceutical manufacturing and a few other permitted uses. The cost-benefit approach favoured by lawmakers has been criticised,94 but the latest ruling on methylene chloride is just a continuation of a recent trend of international regulatory action against solvents with chronic toxicity hazards.Chemists and chemical engineers need to avoid developing products and processes using methylene chloride to avoid being trapped into a reliance on substances subject to, or at risk of, regulatory interventions. The major uses of methylene chloride across formulations, separations, and reactions all have promising alternatives if practitioners are willing to adapt protocols and this transition to safer solvents should start in the teaching laboratory.95,96 Ethyl acetate and dimethyl carbonate recur as promising substitutes across different case studies with safer environmental health and safety profiles (see ESI Fig. S1†). To discover the optimum solvent in any application, it is essential to understand the role of the solvent through experiment, but it would be pertinent to first try either ethyl acetate or dimethyl carbonate in place of methylene chloride before undertaking more thorough redesigns of products or processes to accommodate other solvents. Many sectors have enthusiastically taken to the challenge of eliminating methylene chloride,97 and this latest risk management rule will make others follow suit.
Data availability
The data supporting this article (i.e. literature survey results) have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.References
- M. Rossberg in Ullmann's Encyclopedia of Industrial Chemistry, ed. W. Gerhartz, Wiley VCH, Weinheim, Chloromethanes, 5th edn, 1986, pp. 233–236 Search PubMed.
- T. Anthony in Kirk-Othmer Encyclopedia of Chemical Technology, ed. M. Grayson, John Wiley and Sons, New York, 3rd edn, 1978, Chlorocarbons, -hydrocarbons (CH2Cl2), pp. 235–236 Search PubMed.
- United States Environmental Protection Agency, Release Reports, https://enviro.epa.gov/triexplorer/tri_release.chemical, 2025 Search PubMed.
- A. Jordan, P. Stoy and H. F. Sneddon, Chlorinated solvents: their advantages, disadvantages, and alternatives in organic and medicinal chemistry, Chem. Rev., 2021, 121, 1582, DOI:10.1021/acs.chemrev.0c00709.
- G. Cavallo, P. Metrangolo, R. Milani, T. Pilati, A. Priimagi, G. Resnati and G. Terraneo, The halogen bond, Chem. Rev., 2016, 116, 2478, DOI:10.1021/acs.chemrev.5b00484.
- United States Environmental Protection Agency, Risk Management for Methylene Chloride, https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/risk-management-methylene-chloride, 2025.
- L. Benbrahim-Tallaa, B. Lauby-Secretan, D. Loomis, K. Z. Guyton, Y. Grosse, F. El Ghissassi, V. Bouvard, N. Guha, H. Mattock and K. Straif, Carcinogenicity of perfluorooctanoic acid, tetrafluoroethylene, dichloromethane, 1,2-dichloropropane, and 1,3-propane sultone, Lancet Oncol., 2014, 15, 924, DOI:10.1016/S1470-2045(14)70316-X.
- United States Government, Risk Evaluation for Methylene Chloride, https://www.regulations.gov/document/EPA-HQ-OPPT-2019-0437-0107, 2025.
- United States Government: Methylene Chloride, Regulation Under the Toxic Substances Control Act (TSCA). Federal Register, 2024, 89 (90), 39254–39302, https://www.govinfo.gov/content/pkg/FR-2024-05-08/pdf/2024-09606.pdf.
- United States Environmental Protection Agency, A Guide to Complying with the 2024 Methylene Chloride Regulation under the Toxic Substances Control Act, https://www.epa.gov/system/files/documents/2024-07/mecl-compliance-guide.pdf, 2025.
- L. Goulding, The Gist of the List, ACS Chem. Health Saf., 2024, 31, 272, DOI:10.1021/acs.chas.4c00053.
- United States Environmental Protection Agency, Ongoing and Completed Chemical Risk Evaluations under TSCA, https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/ongoing-and-completed-chemical-risk-evaluations-under, 2025.
- J. Sherwood, European restrictions on 1,2-dichloroethane: C–H activation research and development should be liberated and not limited, Angew. Chem., Int. Ed., 2018, 57, 14286, DOI:10.1002/anie.201800549.
- European Chemicals Agency: Substances restricted under REACH, https://echa.europa.eu/substances-restricted-under-reach/-/dislist/details/0b0236e1807e2f57, 2025.
- European Commission, Safe and Sustainable by Design, https://research-and-innovation.ec.europa.eu/research-area/industrial-research-and-innovation/chemicals-and-advanced-materials/safe-and-sustainable-design_en, 2025.
- K. Alfonsi, J. Colberg, P. J. Dunn, T. Fevig, S. Jennings, T. A. Johnson, H. P. Kleine, C. Knight, M. A. Nagy, D. A. Perry and M. Stefaniak, Green chemistry tools to influence a medicinal chemistry and research chemistry based organisation, Green Chem., 2008, 10, 31, 10.1039/B711717E.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, CHEM21 selection guide of classical- and less classical-solvents, Green Chem., 2016, 18, 288, 10.1039/C5GC01008J.
- A. Hoang, K. Fagan, D. L. Cannon, S. D. G. Rayasam, R. Harrison, D. Shusterman and V. Singla, Assessment of methylene chloride–related fatalities in the United States, 1980-2018, JAMA Intern. Med., 2021, 181, 797, DOI:10.1001/jamainternmed.2021.1063.
- J. B. Leikin, D. Kaufman, J. W. Lipscomb, M. A. Burda and D. O. Hryhorczuk, Methylene chloride: report of five exposures and two deaths, Am. J. Emerg. Med., 1990, 8, 534, DOI:10.1016/0735-6757(90)90158-V.
- R. Shang, Q. Tian, Y. Liu, H. Liu, X. Zhang, M. Shi, X. Jian and Q. Li, Case report: sudden death caused by methylene chloride poisoning, Front. Pharmacol, 2024, 15, 1471744, DOI:10.3389/fphar.2024.1471744.
- S. Vidal, Safety first: a recent case of a dichloromethane injection injury, ACS Cent. Sci., 2020, 6, 83, DOI:10.1021/acscentsci.0c00100.
- R. E. Conrow and W. D. Dean, Diazidomethane explosion, Org. Process Res. Dev., 2008, 12, 1285, DOI:10.1021/op8000977.
- United Nations Environment Programme, The Montreal Protocol on Substances that Deplete the Ozone Layer, https://ozone.unep.org/treaties/montreal-protocol, 2025.
- R. Hossaini, M. P. Chipperfield, S. A. Montzka, A. A. Leeson, S. S. Dhomse and J. A. Pyle, The increasing threat to stratospheric ozone from dichloromethane, Nat. Commun., 2017, 8, 15962, DOI:10.1038/ncomms15962.
- M. An, L. M. Western, D. Say, L. Chen, T. Claxton, A. Ganesan, R. Hossaini, P. B. Krummel, A. J. Manning, J. Mühle, S. O'Doherty, R. G. Prinn, R. F. Weiss, D. Young, J. Hu, B. Yao and M. Rigby, Rapid increase in dichloromethane emissions from China inferred through atmospheric observations, Nat. Commun., 2021, 12, 7279, DOI:10.1038/s41467-021-27592-y.
- European Chemicals Agency, Annex XVII to REACH – Conditions of restriction, https://echa.europa.eu/documents/10162/0ea58491-bb76-4a47-b1d2-36faa1e0f290, 2025.
- United States Government, Regulation of Paint and Coating Removal for Consumer Use: Methylene Chloride, https://www.regulations.gov/document/EPA-HQ-OPPT-2016-0231-0980, 2025.
- S. Ayad, Z.-E. Lin, P.-T. Chiueh, S. Suh, D.-Q. Liu and M. Lee, Turning regrettable substitutions into informed choices: empowering chemical alternative assessment through life cycle perspective, J. Clean. Prod., 2025, 489, 144689, DOI:10.1016/j.jclepro.2025.144689.
- R. A. Milescu, T. J. Farmer, J. Sherwood, C. R. McElroy and J. H. Clark, Cyrene™, a sustainable solution for graffiti paint removal, Sustainable Chem., 2023, 4, 154, DOI:10.3390/suschem4020012.
- C. P. Ashcroft, P. J. Dunn, J. D. Hayler and A. S. Wells, Survey of solvent usage in papers published in organic process research & development 1997–2012, Org. Process Res. Dev., 2015, 19, 740, DOI:10.1021/op500276u.
- J. P. Taygerly, L. M. Miller, A. Yee and E. A. Peterson, A convenient guide to help select replacement solvents for dichloromethane in chromatography, Green Chem., 2012, 14, 3020, 10.1039/C2GC36064K.
- D. S. MacMillan, J. Murray, H. F. Sneddon, C. Jamieson and A. J. B. Watson, Replacement of dichloromethane within chromatographic purification: a guide to alternative solvents, Green Chem., 2012, 14, 3016, 10.1039/C2GC36378J.
- C. Ayafor, T. Burton, N. George, G. Morose and H.-W. Wong, Safer solvents for active pharmaceutical ingredient purification using column chromatography, ACS Environ. Au, 2024, 4, 236, DOI:10.1021/acsenvironau.4c00015.
- J. Lynch, J. Sherwood, C. R. McElroy, J. Murray and S. Shimizu, Dichloromethane replacement: towards greener chromatography via Kirkwood–Buff integrals, Anal. Methods, 2023, 15, 596, 10.1039/D2AY01266A.
- A. Sharma, E. Yu, G. Morose, D. T. Nguyen and W.-T. Chen, Designing safer solvents to replace methylene chloride for liquid chromatography applications using thin-layer chromatography as a screening tool, Separations, 2021, 8, 172, DOI:10.3390/separations8100172.
- E. A. Peterson, B. Dillon, I. Raheem, P. Richardson, D. Richter, R. Schmidt and H. F. Sneddon, Sustainable chromatography (an oxymoron?), Green Chem., 2014, 16, 4060, 10.1039/C4GC00615A.
- A. F. Diefendorf, Comparing lipid extraction methods on lake sediments without dichloromethane, Org. Geochem., 2025, 202, 104919, DOI:10.1016/j.orggeochem.2024.104919.
- I. Smallwood, Handbook of Organic Solvent Properties, Butterworth-Heinemann, Oxford, 1996 Search PubMed.
- Z. Shi, G. B. Hammond and B. Xu, Faster and greener chemical reaction workup using silicone elastomer-coated glass powders, ACS Omega, 2018, 3, 6748, DOI:10.1021/acsomega.8b00966.
- T. Li and B. Xu, Faster and greener parallel chemical reaction work-up using ‘sponge’ extraction, Tetrahedron Lett., 2022, 95, 153702, DOI:10.1016/j.tetlet.2022.153702.
- N. Cayot, C. Lafarge, E. Bou-Maroun and P. Cayot, Substitution of carcinogenic solvent dichloromethane for the extraction of volatile compounds in a fat-free model food system, J. Chromatogr. A, 2016, 1456, 77, DOI:10.1016/j.chroma.2016.06.015.
- D. V. Bermejo, J. A. Mendiola, E. Ibáñez, G. Reglero and T. Fornari, Pressurized liquid extraction of caffeine and catechins from green tea leaves using ethyl lactate, water and ethyl lactate + water mixtures, Food Bioprod. Process., 2015, 96, 106, DOI:10.1016/j.fbp.2015.07.008.
- I. De Marco, S. Riemma and R. Iannone, Life cycle assessment of supercritical CO2 extraction of caffeine from coffee beans, J. Supercrit. Fluids, 2018, 133, 393, DOI:10.1016/j.supflu.2017.11.005.
- United States Government, Secondary Direct Food Additives Permitted in Food for Human Consumption, https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-173/subpart-C/section-173.255, 2025.
- European Union, The Approximation of the Laws of the Member States on Extraction Solvents used in the Production of Foodstuffs and Food Ingredients, https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:02009L0032-20100916, 2025.
- D. Prat, J. Hayler and A. Wells, A survey of solvent selection guides, Green Chem., 2014, 16, 4546, 10.1039/C4GC01149J.
- D. J. C. Constable, C. Jimenez-Gonzalez and R. K. Henderson, Perspective on solvent use in the pharmaceutical industry, Org. Process Res. Dev., 2007, 11, 133, DOI:10.1021/op060170h.
- C. R. Roelofs and M. J. Ellenbecker, Source reduction for prevention of methylene chloride hazards: cases from four industrial sectors, Environ. Health, 2003, 2, 9, DOI:10.1186/1476-069X-2-9.
- Sygnature Discovery: Reducing DCM use, https://www.sygnaturediscovery.com/publications/posters/reducing-dcm-use/, 2025.
- J. H. Clark, D. J. Macquarrie and J. Sherwood, A quantitative comparison between conventional and bio-derived solvents from citrus waste in esterification and amidation kinetic studies, Green Chem., 2012, 14, 90, 10.1039/C1GC16299C.
- D. Procopio, C. Siciliano, S. Trombino, D. E. Dumitrescu, F. Suciu and M. L. Di Gioia, Green solvents for the formation of amide linkages, Org. Biomol. Chem., 2022, 20, 1137, 10.1039/D1OB01814K.
- L. A. Carpino, 1-Hydroxy-7-azabenzotriazole. An efficient peptide coupling additive, J. Am. Chem. Soc., 1993, 115, 4397, DOI:10.1021/ja00063a082.
- K. L. Wilson, J. Murray, C. Jamieson and A. J. B. Watson, Cyrene as a bio-based solvent for HATU mediated amide coupling, Org. Biomol. Chem., 2018, 16, 2851, 10.1039/C8OB00653A.
- T. W. Bousfield, K. P. R. Pearce, S. B. Nyamini, A. Angelis-Dimakis and J. E. Camp, Synthesis of amides from acid chlorides and amines in the bio-based solvent Cyrene, Green Chem., 2019, 21, 3675, 10.1039/C9GC01180C.
- S. B. Lawrenson, R. Arav and M. North, The greening of peptide synthesis, Green Chem., 2017, 19, 1685, 10.1039/C7GC00247E.
- G. L. Beutner, I. S. Young, M. L. Davies, M. R. Hickey, H. Park, J. M. Stevens and Q. Ye, TCFH-NMI: direct access to N-acyl imidazoliums for challenging amide bond formations, Org. Lett., 2018, 20, 4218, DOI:10.1021/acs.orglett.8b01591.
- A. El-Faham, R. S. Funosas, R. Prohens and F. Albericio, COMU: a safer and more effective replacement for benzotriazole-based uronium coupling reagents, Chem. –Eur. J., 2009, 15, 9404, DOI:10.1002/chem.200900615.
- D. S. MacMillan, J. Murray, H. F. Sneddon, C. Jamieson and A. J. B. Watson, Evaluation of alternative solvents in common amide coupling reactions: replacement of dichloromethane and N,N-dimethylformamide, Green Chem., 2013, 15, 596, 10.1039/C2GC36900A.
- K. M. Freiberg, R. D. Kavthe, R. M. Thomas, D, M. Fialho, P. Dee, M. Scurria and B. H. Lipshutz, Direct formation of amide/peptide bonds from carboxylic acids: no traditional coupling reagents, 1-pot, and green, Chem. Sci., 2023, 14, 3462, 10.1039/D3SC00198A.
- S. Hazra, F. Gallou and S. Handa, Water: an underestimated solvent for amide bond-forming reactions, ACS Sustainable Chem. Eng., 2022, 10, 5299, DOI:10.1021/acssuschemeng.2c00520.
- D. Procopio, C. Siciliano and M. L. Di Gioia, Reactive deep eutectic solvents for EDC-mediated amide synthesis, Org. Biomol. Chem., 2024, 22, 1400, 10.1039/D3OB01673K.
- W. I. Nicholson, F. Barreteau, J. A. Leitch, R. Payne, I. Priestley, E. Godineau, C. Battilocchio and D. L. Browne, Direct amidation of esters by ball milling, Angew. Chem., Int. Ed., 2021, 60, 21868, DOI:10.1002/anie.202106412.
- R. Varala, S. Nuvula and S. R. Adapa, Molecular iodine-catalyzed facile procedure for N-Boc protection of amines, J. Org. Chem., 2006, 71, 8283, DOI:10.1021/jo0612473.
- J. C. Graham, A. Trejo-Martin, M. L. Chilton, J. Kostal, J. Bercu, G. L. Beutner, U. S. Bruen, D. G. Dolan, S. Gomez, J. Hillegass, J. Nicolette and M. Schmitz, An evaluation of the occupational health hazards of peptide couplers, Chem. Res. Toxicol., 2022, 35, 1011, DOI:10.1021/acs.chemrestox.2c00031.
- M. Lubberink, W. Finnigan and S. L. Flitsch, Biocatalytic amide bond formation, Green Chem., 2023, 25, 2958, 10.1039/D3GC00456B.
- M. T. Sabatini, L. T. Boulton, H. F. Sneddon and T. D. Sheppard, A green chemistry perspective on catalytic amide bond formation, Nat. Catal., 2019, 2, 10, DOI:10.1038/s41929-018-0211-5.
- H. P. H. Arp, A. Gredelj, J. Glüge, M. Scheringer and I. T. Cousins, The global threat from the irreversible accumulation of trifluoroacetic acid (TFA), Environ. Sci. Technol., 2024, 58, 19925, DOI:10.1021/acs.est.4c06189.
- A. R. Bogdan, M. Charaschanya, A. W. Dombrowski, Y. Wang and S. W. Djuric, High-temperature Boc deprotection in flow and its application in multistep reaction sequences, Org. Lett., 2016, 18, 1732, DOI:10.1021/acs.orglett.6b00378.
- B. Li, R. Li, P. Dorff, J. C. McWilliams, R. M. Guinn, S. M. Guinness, L. Han, K. Wang and S. Yu, Deprotection of N-Boc groups under continuous-flow high-temperature conditions, J. Org. Chem., 2019, 84, 4846, DOI:10.1021/acs.joc.8b02909.
- G. Wang, C. Li, J. Li and X. Jia, Catalyst-free water-mediated N-Boc deprotection, Tetrahedron Lett., 2009, 50, 1438, DOI:10.1016/j.tetlet.2009.01.056.
- J. Wang, Y.-L. Liang and J. Qu, Boiling water-catalyzed neutral and selective N-Boc deprotection, Chem. Commun., 2009, 5144, 10.1039/B910239F.
- R. H. Verschueren, P. Gilles, S. Van Mileghem and W. M. De Borggraeve, Solvent-free N-Boc deprotection by ex situ generation of hydrogen chloride gas, Org. Biomol. Chem., 2021, 19, 5782, 10.1039/D1OB00728A.
- N. George, S. Ofori, S. Parkin and S. G. Awuah, Mild deprotection of the N-tert-butyloxycarbonyl (N-Boc) group using oxalyl chloride, RSC Adv., 2020, 10, 24017, 10.1039/D0RA04110F.
- J. H. Clark, E. M. Fitzpatrick, D. J. Macquarrie, L. A. Pfaltzgraff and J. Sherwood, p-Cymenesulphonic acid: an organic acid synthesised from citrus waste, Catal. Today, 2012, 190, 144, DOI:10.1016/j.cattod.2011.12.007.
- A. Jordan, K. D. Whymark, J. Sydenham and H. F. Sneddon, A solvent-reagent selection guide for Steglich-type esterification of carboxylic acids, Green Chem., 2021, 23, 6405, 10.1039/D1GC02251B.
- Y. Li, H. Kuang, Z. Shen, G. Bao and W. Sun, Fast esterification method mediated by coupling reagent NDTP, ACS Omega, 2025, 10, 8113, DOI:10.1021/acsomega.4c09365.
- A. Jordan and H. F. Sneddon, Development of a solvent-reagent selection guide for the formation of thioesters, Green Chem., 2019, 21, 1900, 10.1039/C9GC00355J.
- N. R. Luis, K. K. Chung, M. R. Hickey, Z. Lin, G. L. Beutner and D. A. Vosburg, Beyond amide bond formation: TCFH as a reagent for esterification, Org. Lett., 2024, 26, 2745, DOI:10.1021/acs.orglett.3c01611.
- A. Jordan, C. G. J. Hall, L. R. Thorp and H. F. Sneddon, Replacement of less-preferred dipolar aprotic and ethereal solvents in synthetic organic chemistry with more sustainable alternatives, Chem. Rev., 2022, 122, 6749, DOI:10.1021/acs.chemrev.1c00672.
- X. Deng and N. S. Mani, A facile, environmentally benign sulfonamide synthesis in water, Green Chem., 2006, 8, 835, 10.1039/B606127C.
- N. Eid, I. Karamé and B. Andrioletti, Straightforward and sustainable synthesis of sulfonamides in water under mild conditions, Eur. J. Org Chem., 2018, 5016, DOI:10.1002/ejoc.201800504.
- M. Simone, M. Pulpito, F. M. Perna, V. Capriati and P. Vitale, Switchable deep eutectic solvents for sustainable sulfonamide synthesis, Chem. - Eur. J., 2024, 30, e202402293, DOI:10.1002/chem.202402293.
- N. Mostajeran, F. A. Arshad, H. Aliyan and A. R. Massah, Solvent-free synthesis and antibacterial evaluation of novel coumarin sulfonamides, Pharm. Chem. J., 2018, 52, 1, DOI:10.1007/s11094-018-1756-y.
- F. I. McGonagle, D. S. MacMillan, J. Murray, H. F. Sneddon, C. Jamieson and A. J. B. Watson, Development of a solvent selection guide for aldehyde-based direct reductive amination processes, Green Chem., 2013, 15, 1159, 10.1039/C3GC40359A.
- J. F. W. McOmie, M. L. Watts and D. E. West, Demethylation of aryl methyl ethers by boron tribromide, Tetrahedron, 1968, 24, 2289, DOI:10.1016/0040-4020(68)88130-X.
- D. Sang, J. Tian, X. Tu, Z. He and M. Yao, Cleavage of catechol monoalkyl ethers by aluminum triiodide–dimethyl sulfoxide, Synthesis, 2019, 51, 704, DOI:10.1055/s-0037-1610996.
- R. Bernini, M. Barontini, P. Mosesso, G. Pepe, S. M. Willför, R. E. Sjöholm, P. C. Eklund and R. Saladino, A selective de-O-methylation of guaiacyl lignans to corresponding catechol derivatives by 2-iodoxybenzoic acid (IBX). The role of the catechol moiety on the toxicity of lignans, Org. Biomol. Chem., 2009, 7, 2367, 10.1039/B822661J.
- S. Pompei, C. Grimm, C. Schiller, L. Schober and W. Kroutil, Thiols act as methyl traps in the biocatalytic demethylation of guaiacol derivatives, Angew. Chem., Int. Ed., 2021, 60, 16906, DOI:10.1002/anie.202104278.
- A. R. Podilapu and S. S. Kulkarni, First synthesis of bacillus cereus Ch HF-PS cell wall trisaccharide repeating unit, Org. Lett., 2014, 16, 4336, DOI:10.1021/ol5021527.
- W. Qu, S. Oya, B. P. Lieberman, K. Ploessl, L. Wang, D. R. Wise, C. R. Divgi, L. P. Chodosh, C. B. Thompson and H. F. Kung, Preparation and characterization of l-[5-11C]-glutamine for metabolic imaging of tumors, J. Nucl. Med., 2012, 53, 98, DOI:10.2967/jnumed.111.093831.
- O. B. Andrew, J. Sherwood and G. A. Hurst, A greener synthesis of the antidepressant bupropion hydrochloride, J. Chem. Educ., 2022, 99, 3277, DOI:10.1021/acs.jchemed.2c00581.
- K. Skowerski, J. Białecki, A. Tracz and T. K. Olszewski, An attempt to provide an environmentally friendly solvent selection guide for olefin metathesis, Green Chem., 2014, 16, 1125, 10.1039/C3GC41943F.
- R. M. Ward, Y. Hu, N. P. Tu and J. M. Schomaker, Solvent effects on the chemo- and site-selectivity of transition metal-catalyzed nitrene transfer reactions: alternatives to chlorinated solvents, ChemSusChem, 2024, 17, e202300964, DOI:10.1002/cssc.202300964.
- J. Goodwin and F. Holm, Methylene chloride comment, New Solut., 2023, 33, 174, DOI:10.1177/10482911231198148.
- A. Milo, L. Chen, K. A. Grice and D. A. Vosburg, Alternatives to Dichloromethane for Teaching Laboratories, J. Chem. Educ., 2025, 102, 2261, DOI:10.1021/acs.jchemed.5c00106.
- S. W. Wright and C. O. Welder, Elimination of Dichloromethane from the Introductory Organic Chemistry Teaching Laboratory, J. Chem. Educ., 2025, 102, 2845–2851, DOI:10.1021/acs.jchemed.5c00430.
- R. Yogesh, N. Srivastava and B. M. Mulik, Efforts to replace methylene chloride in pharmaceutical process chemistry, Macromol. Symp., 2023, 407, 2100502, DOI:10.1002/masy.202100502.
Footnote |
| † Electronic supplementary information (ESI) available: Literature survey and reaction frequency analysis. See DOI: https://doi.org/10.1039/d5su00443h |
| This journal is © The Royal Society of Chemistry 2025 |





