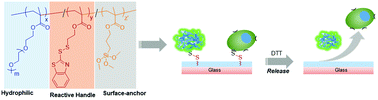
Selective modification of polymeric coatings with high fidelity is important for various biomedical applications. This is especially true if reversible functionalization is desired to achieve a conditional response. In the present study, we report on the synthesis of functional copolymers bearing activated disulfide groups as side chains that are amenable to reversible thiol-based functionalization. A methacrylic monomer carrying a redox-responsive benzothiazole-disulfide group was copolymerized with a hydrophilic polyethylene glycol (PEG) based monomer using reversible addition–fragmentation chain transfer (RAFT) polymerization. Functional copolymers with varying ratios of the benzothiazole disulfide-based methacrylate (BDSMA) monomer were obtained with high conversions and narrow molecular weight distributions. The obtained copolymers were demonstrated to undergo facile post-polymerization functionalization through the thiol–disulfide exchange reaction with model thiol-containing molecules. These polymers were employed in the fabrication of reactive surface coatings on glass, where functionalization using thiol-containing fluorescent dyes and cell-adhesive ligands was demonstrated. The reversibility of the reaction enabled the release of the conjugated molecules upon exposure to dithiothreitol (DTT). Redox-responsive disulfide conjugation of cell-adhesive peptides enabled attachment of cells on the surface, and their subsequent release under a reducing environment. Facile synthesis of these efficiently functionalizable disulfide-containing copolymers and their utilization to fabricate reversibly functionalizable coatings provide a versatile interface for various biomedical applications.