Chihiro Tsukano, Motoyuki Nakajima, Sudhir M. Hande and Yoshiji Takemoto
Org. Biomol. Chem., 2019,17, 1731-1735
DOI:
10.1039/C8OB02224K,
Communication
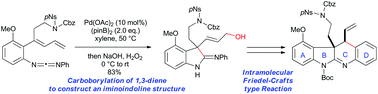
A palladium-catalyzed intramolecular carboborylation of 1,3-diene has been developed for the synthesis of iminoindolines with a quaternary carbon centre. This method was applied to a substrate bearing several functional groups to afford a complex iminoindoline, which was subsequently converted into an ABCD ring model compound of communesins via an intramolecular Friedel–Crafts-type reaction.