Sara Cuadros, Matthew A. Horwitz, Bertrand Schweitzer-Chaput and Paolo Melchiorre
Chem. Sci., 2019,10, 5484-5488
DOI:
10.1039/C9SC00833K,
Edge Article
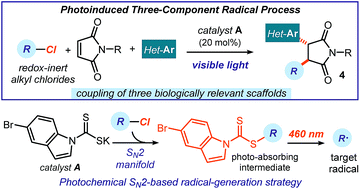
We report a photoinduced three-component radical process, which couples readily available alkyl chlorides, maleimides, and heteroaromatic fragments to rapidly generate complex chiral products with high diastereocontrol. This method generates radicals via an SN2-based photochemical catalytic mechanism, which is not reliant on the redox properties of the precursors. It therefore grants access to open-shell intermediates from substrates that would be incompatible with or inert to classical radical-generating strategies. The redox-neutral conditions of this process make it tolerant of redox-sensitive substrates and allow the installation of multiple biologically relevant heterocycles within the cascade products.