Magnetic properties of polynuclear complexes. Part II. Superexchange in some binuclear cobalt(II) complexes
Abstract
The average magnetic susceptibilities of six binuclear complexes of cobalt(II) between 80 and 400 K are reported. The compounds studied are Co2(dhph)2X4,nH2O (dhph = 1,4-dihydrazinophthalazine, X = Cl or Br), Co2(dppn)2X4,nH2O [dppn = 3,6-di-(2-pyridyl)pyridazine, X = NO3 or ClO4], Co2(dppn)(SO4)2,5H2O, and Co2(Me2dppn)(NO3)4,2CH3OH [Me2dppn = 3,6-di-(6-methyl-2-pyridyl)pyridazine]. These compounds exhibit weak antiferromagnetic exchange, and the values of the effective isotropic exchange parameter 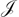 are estimated, making allowance for spin–orbit coupling and the effects of orbital reduction and axial distortion parameters. The values of
are estimated, making allowance for spin–orbit coupling and the effects of orbital reduction and axial distortion parameters. The values of 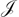 are compared with those of the analogous nickel complexes reported earlier, and it is concluded that the t2g spin of Co2+ probably makes a ferromagnetic contribution. The exchange interaction is analysed in a one-electron orbital basis, leading to an anisotropic form for the exchange parameter. The average susceptibility data do not distinguish between the isotropic and anisotropic models, however, and the precise identity of the orbitals responsible for the ferromagnetic contribution remains uncertain.
are compared with those of the analogous nickel complexes reported earlier, and it is concluded that the t2g spin of Co2+ probably makes a ferromagnetic contribution. The exchange interaction is analysed in a one-electron orbital basis, leading to an anisotropic form for the exchange parameter. The average susceptibility data do not distinguish between the isotropic and anisotropic models, however, and the precise identity of the orbitals responsible for the ferromagnetic contribution remains uncertain.

 Please wait while we load your content...
Please wait while we load your content...