The nucleophilicity N index (J. Org. Chem.2008, 73, 4615), the inverse of the electrophilicity, 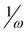 , and the recently proposed inverse of the electrodonating power,
, and the recently proposed inverse of the electrodonating power, 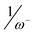 , (J. Org. Chem.2010, 75, 4957) have been checked toward (i) a series of single 5-substituted indoles for which rate constants are available, (ii) a series of para-substituted phenols, and for (iii) a series of 2,5-disubstituted bicyclic[2.2.1]hepta-2,5-dienes which display concurrently electrophilic and nucleophilic behaviors. While all considered indices account well for the nucleophilic behavior of organic molecules having a single substitution, the nucleophilicity N index works better for more complex molecules. Unlike, the inverse of the electrophilicity,
, (J. Org. Chem.2010, 75, 4957) have been checked toward (i) a series of single 5-substituted indoles for which rate constants are available, (ii) a series of para-substituted phenols, and for (iii) a series of 2,5-disubstituted bicyclic[2.2.1]hepta-2,5-dienes which display concurrently electrophilic and nucleophilic behaviors. While all considered indices account well for the nucleophilic behavior of organic molecules having a single substitution, the nucleophilicity N index works better for more complex molecules. Unlike, the inverse of the electrophilicity, 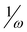 , (R2 = 0.71), and the inverse of the electrodonating power,
, (R2 = 0.71), and the inverse of the electrodonating power, 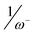 (R2 = 0.83), a very good correlation of the nucleophilicity N index of twelve 2-substituted-6-methoxy-bicyclic[2.2.1]hepta-2,5-dienes versus the activation energy associated with the nucleophilic attack on 1,1-dicyanoethylene is found (R2 = 0.99). This comparative study allows to assert that the nucleophilicity N index is a measure of the nucleophilicity of complex organic molecules displaying concurrently electrophilic and nucleophilic behaviors.
(R2 = 0.83), a very good correlation of the nucleophilicity N index of twelve 2-substituted-6-methoxy-bicyclic[2.2.1]hepta-2,5-dienes versus the activation energy associated with the nucleophilic attack on 1,1-dicyanoethylene is found (R2 = 0.99). This comparative study allows to assert that the nucleophilicity N index is a measure of the nucleophilicity of complex organic molecules displaying concurrently electrophilic and nucleophilic behaviors.
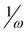 , and the recently proposed inverse of the electrodonating power,
, and the recently proposed inverse of the electrodonating power, 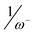 , (J. Org. Chem.2010, 75, 4957) have been checked toward (i) a series of single 5-substituted
, (J. Org. Chem.2010, 75, 4957) have been checked toward (i) a series of single 5-substituted 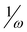 , (R2 = 0.71), and the inverse of the electrodonating power,
, (R2 = 0.71), and the inverse of the electrodonating power, 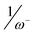 (R2 = 0.83), a very good correlation of the nucleophilicity N index of twelve 2-substituted-6-methoxy-bicyclic[2.2.1]hepta-2,5-dienes versus the activation energy associated with the nucleophilic attack on
(R2 = 0.83), a very good correlation of the nucleophilicity N index of twelve 2-substituted-6-methoxy-bicyclic[2.2.1]hepta-2,5-dienes versus the activation energy associated with the nucleophilic attack on 

 Please wait while we load your content...
Please wait while we load your content...