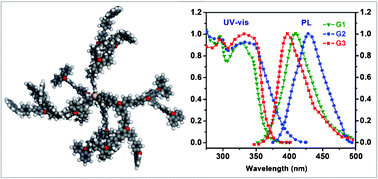
Three generations of novel dendrimers (G1, G2 and G3) with a tetraphenylsilane core and 9-phenylcarbazole-based dendrons have been successfully synthesized. These dendrimers possess excellent thermal stability with 5% weight-loss temperatures in the range of 574 to 622 °C. Compared to the lower generation of dendrimer, G3 exhibits a high glass transition at 271 °C, indicating that the high content of 9-phenylcarbazole results in a dendrimer with significantly enhanced rigidity. The contorted dendrons effectively inhibit the aggregation of conjugative chromophore groups, leading to a fluorescence quantum yield of up to 99.1%. Moreover, all the dendrimers have high energy band gaps, a characteristic which is especially desirable for host materials in the fabrication of organic light-emitting devices.