Low viscosity highly conductive ionic liquid blends for redox active electrolytes in efficient dye-sensitized solar cells
Abstract
Mixtures of ionic liquids were prepared and used for the development of composite redox electrolytes by blending a standard low viscosity ionic liquid solvent (EMimDCA, 1-ethyl-3-methylimidazolium dicyanamide) with various iodide-based ionic liquids based on the methylimidazolium cation (DMII, EMII, PMII, BMII and HMII). The novel electrolytes based on the [CnC1im]I–EMimDCA double salt ILs show interesting physicochemical properties including low viscosity (10–110 MPa s) and high diffusion coefficient of triiodides 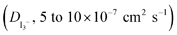 , respectively, characteristics that promise increased performance in DSC devices. Their electrochemical properties along with the conductivity were also tuned and optimized; values as high as 2–4 mS cm−1 were estimated for the conductivity. Solar cells based on these composite electrolytes attained efficiencies over 4% under 1 sun with the highest being 5.5%, attained by the EMimDCA–DMII mixture. Quite notably, these efficiencies further increased up to 6.5%, when the cells were illuminated by 0.1 sun.
, respectively, characteristics that promise increased performance in DSC devices. Their electrochemical properties along with the conductivity were also tuned and optimized; values as high as 2–4 mS cm−1 were estimated for the conductivity. Solar cells based on these composite electrolytes attained efficiencies over 4% under 1 sun with the highest being 5.5%, attained by the EMimDCA–DMII mixture. Quite notably, these efficiencies further increased up to 6.5%, when the cells were illuminated by 0.1 sun.


 Please wait while we load your content...
Please wait while we load your content...