RAFT synthesis of well-defined PVDF-b-PVAc block copolymers†
Abstract
RAFT polymerization of vinylidene fluoride (VDF), leading to relatively well defined poly(vinylidene fluoride) (PVDF), is negatively affected by chain inversion resulting in less easily reactivatable PVDFT-XA dormant chains (terminated with the tail end of an inversely added VDF unit; XA = xanthate moiety). Although slow reactivation of these chains by PVDF˙ radicals (in contrast to general belief) was recently demonstrated, slow radical exchange leads to progressive loss of chain growth control. This article deals with the possibility of synthesizing block copolymers from PVDF-XA macroCTAs by sequential addition. The investigations show that only PVDFH-XA (chains terminated with the head end of regularly added VDF) can be reactivated by PNVP˙ (poly(N-vinylpyrrolidone)) radicals and that PVDFT-XA chains are completely unreactive in the presence of PNVP˙, PB˙ (poly(butylacrylate)) or PDM˙ (poly(N,N′-dimethylacrylamide)). However, both PVDFH-XA and PVDFT-XA can be reactivated by PVAc˙ (poly(vinyl acetate)) radicals. The reactivation of the PVDFT-XA, albeit slower than that of the PVDFH-XA, is sufficiently fast to allow the synthesis of unprecedented well-defined PVDF-b-PVAc block copolymers with relatively high end-group fidelity. DFT calculations rationalize this behavior on the basis of faster radical exchange in the order PVDFH-XA/VAc > PVDFH-XA/NVP > PVDFT-XA/VAc ≫ PVDFT-XA/NVP. The success of the chain extension also relies on faster activation relative to homopropagation of the chain extending monomer, as well as fast addition of the released 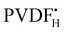 and
and 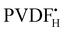 to the monomer.
to the monomer.


 Please wait while we load your content...
Please wait while we load your content...