Carbon nitride quantum dot-based chemiluminescence resonance energy transfer for iodide ion sensing†
Abstract
In this study, a dramatically enhanced chemiluminescence (CL) was observed in Ce(IV) and sulfite system in the presence of graphitic carbon nitride quantum dots (g-CNQDs). On the basis of CL spectra, UV-vis absorption, fluorescence (FL), Fourier transform infrared spectroscopy (FTIR), X-ray photoelectron spectroscopy (XPS) and electron paramagnetic resonance (EPR) and the effect of various free radical scavengers, a possible CL mechanism of radiative recombination of the holes-injected and electrons-injected g-CNQDs was suggested to account for the surprising g-CNQD CL behavior. Meanwhile, the excited sulfur dioxide molecules 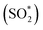 , produced from the interaction between Ce(IV) and sulfite under acidic conditions, could transfer energy to g-CNQDs and further enhance the CL emission. In addition, the CL was dependent on the FL quantum yields of g-CNQDs with different surface states, since the chemiluminescence resonance energy transfer (CRET) efficiency was affected by the FL quantum yields of the g-CNQDs. The designed CL system was successfully applied to determine I− in urine samples with good recoveries. It is anticipated that g-CNQDs could be a new class of CRET receptors for fabricating CL sensors. These findings would provide new insight into the optical properties of g-CNQDs and further broaden their applications in CL fields.
, produced from the interaction between Ce(IV) and sulfite under acidic conditions, could transfer energy to g-CNQDs and further enhance the CL emission. In addition, the CL was dependent on the FL quantum yields of g-CNQDs with different surface states, since the chemiluminescence resonance energy transfer (CRET) efficiency was affected by the FL quantum yields of the g-CNQDs. The designed CL system was successfully applied to determine I− in urine samples with good recoveries. It is anticipated that g-CNQDs could be a new class of CRET receptors for fabricating CL sensors. These findings would provide new insight into the optical properties of g-CNQDs and further broaden their applications in CL fields.


 Please wait while we load your content...
Please wait while we load your content...