Chemical instability leads to unusual chemical-potential-independent defect formation and diffusion in perovskite solar cell material CH3NH3PbI3
Abstract
Methylammonium (MA) lead triiodide (MAPbI3) has recently emerged as a promising solar cell material. However, MAPbI3 is known to have chemical instability, i.e., MAPbI3 is prone to decomposition into MAI and PbI2 even at moderate temperatures (e.g. 330 K). Here, we show that the chemical instability, as reflected by the calculated negligible enthalpy of formation of MAPbI3 (with respect to MAI and PbI2), has an unusual and important consequence for defect properties, i.e., defect formation energies in low-carrier-density MAPbI3 are nearly independent of the chemical potentials of constituent elements and thus can be uniquely determined. This allows straightforward calculations of defect concentrations and the activation energy of ionic conductivity (the sum of the formation energy and the diffusion barrier of the charged mobile defect) in MAPbI3. The calculated activation energy for ionic conductivity due to 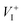 diffusion is in excellent agreement with the experimental values, which demonstrates unambiguously that
diffusion is in excellent agreement with the experimental values, which demonstrates unambiguously that 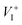 is the dominant diffusing defect and is responsible for the observed ion migration and device polarization in MAPbI3 solar cells. The calculated low formation energy of a Frenkel pair
is the dominant diffusing defect and is responsible for the observed ion migration and device polarization in MAPbI3 solar cells. The calculated low formation energy of a Frenkel pair 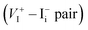 and low diffusion barriers of
and low diffusion barriers of 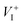 and
and 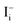 suggest that the iodine ion migration and the resulting device polarization may occur even in single-crystal devices and grain-boundary-passivated polycrystalline thin film devices (which were previously suggested to be free from ion-migration-induced device polarization), leading to device degradation. However, the device polarization due to the Frenkel pair (which has a relatively low concentration) may take a long time to develop and thus may avoid the appearance of the current–voltage hysteresis at typical scan rates.
suggest that the iodine ion migration and the resulting device polarization may occur even in single-crystal devices and grain-boundary-passivated polycrystalline thin film devices (which were previously suggested to be free from ion-migration-induced device polarization), leading to device degradation. However, the device polarization due to the Frenkel pair (which has a relatively low concentration) may take a long time to develop and thus may avoid the appearance of the current–voltage hysteresis at typical scan rates.



 Please wait while we load your content...
Please wait while we load your content...