In situ nuclear magnetic resonance microimaging of live biofilms in a microchannel†
Abstract
Biofilms are comprised of microbial cells and an extracellular polymeric substance (EPS) matrix that supports interactions between community members and with the local environment. The highly hydrated EPS matrix makes the application of many biofilm visualization techniques difficult. Hence, to better visualize how biofilms interact with their environment, there is a need for imaging techniques to monitor hydrated state biofilm dynamics. We employed an in situ dynamic approach to construct label-free images of biofilms. In situ imaging was conducted using a vacuum compatible microfluidic reactor, SALVI (System for Analysis at the Liquid Vacuum Interface), for biofilm growth; real-time confocal laser scanning microscopy analysis; and nuclear magnetic resonance (NMR) microimaging and spectroscopy. We integrated SALVI microchannel fluids and live biofilms to demonstrate in situ measurement capabilities, including velocity mapping, diffusion coefficient mapping, relaxometry, localized spectroscopy, 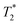 relaxation times, porosity, and two- and three-dimensional imaging within the microchannel at high spatial resolution. We monitored organic acids adjacent to biofilms, suggesting that kinetic rate and substrate-product yield ratio studies are possible using the SALVI microfluidic reactor for growth characterizations. The integration of NMR microimaging studies into the SALVI platform demonstrates that a multimodal microfluidic platform can serve as an avenue to explore complex biological phenomena, such as biofilm attachment to surfaces, with detailed quantitative physical and chemical mapping. The further incorporation of other SALVI-compatible technologies, such as liquid time-of-flight secondary ion mass spectrometry imaging, with NMR microimaging will produce a powerful correlative approach to monitor in situ biofilm chemistry and dynamics at different spatial scales.
relaxation times, porosity, and two- and three-dimensional imaging within the microchannel at high spatial resolution. We monitored organic acids adjacent to biofilms, suggesting that kinetic rate and substrate-product yield ratio studies are possible using the SALVI microfluidic reactor for growth characterizations. The integration of NMR microimaging studies into the SALVI platform demonstrates that a multimodal microfluidic platform can serve as an avenue to explore complex biological phenomena, such as biofilm attachment to surfaces, with detailed quantitative physical and chemical mapping. The further incorporation of other SALVI-compatible technologies, such as liquid time-of-flight secondary ion mass spectrometry imaging, with NMR microimaging will produce a powerful correlative approach to monitor in situ biofilm chemistry and dynamics at different spatial scales.



 Please wait while we load your content...
Please wait while we load your content...