The binuclear dual emitter [Br(CO)3Re(P⋯N)(N⋯P)Re(CO)3Br] (P⋯N): 3-chloro-6-(4-diphenylphosphinyl)butoxypyridazine, a new bridging P,N-bidentate ligand resulting from the ring opening of tetrahydrofuran†‡
Abstract
Lithium diphenylphosphide unexpectedly provokes the ring-opening of tetrahydrofuran (THF) and by reaction with 3,6-dichloropyridazine leads to the formation of the ligand 3-chloro-6-(4-diphenylphosphinyl)butoxypyridazine (P⋯N), which was isolated. The reaction of this ligand with the (Re(CO)3(THF)Br)2 dimer yields the novel complex [Br(CO)3Re(μ-3-chloro-6-(4-diphenylphosphinyl)butoxypyridazine)2Re(CO)3Br] (BrRe(P⋯N)(N⋯P)ReBr), which was crystallized in the form of a chloroform solvate, (C46H40Br2Cl2N4O8P2Re2)·(CHCl3). The monoclinic crystal (P21/n) displays a bimetallic cage structure with a symmetry inversion centre in the middle of the rhenium to rhenium line. The molecule shows two oxidation signals occurring at +1.50 V and +1.76 V which were assigned to the ReI/ReII and ReII/ReIII metal-centered couples, respectively, while signals observed at −1.38 V and −1.68 V were assigned to ligand centered reductions. Experimental and DFT/TDDFT results indicate that the UV-Vis absorption maximum of BrRe(P⋯N)(N⋯P)ReBr occurring near 380 nm displays a metal to ligand charge transfer (MLCT) character, which is consistent with CV results. Upon excitation at this wavelength, a weak emission (Φem < 1 × 10−3) is observed around 580 nm (in dichloromethane) which decays with two distinct lifetimes τ1 and τ2 of 24 and 4.7 ns, respectively. The prevalence of non-radiative deactivation pathways is consistent with efficient internal conversion induced by the high conformational flexibility of the P⋯N ligand's long carbon chain. Measurements in a frozen solvent at 77 K, where vibrational deactivation is hindered, show intense emission associated with the 3MLCT state. These results demonstrate that BrRe(P⋯N)(N⋯P)ReBr preserves the dual emitting nature previously reported for the mononuclear complex RePNBr, with emission associated with 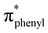 and
and 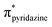 states.
states.
![Graphical abstract: The binuclear dual emitter [Br(CO)3Re(P⋯N)(N⋯P)Re(CO)3Br] (P⋯N): 3-chloro-6-(4-diphenylphosphinyl)butoxypyridazine, a new bridging P,N-bidentate ligand resulting from the ring opening of tetrahydrofuran](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6DT04158B&imageInfo.ImageIdentifier.Year=2017)


 Please wait while we load your content...
Please wait while we load your content...