Decomposition mechanisms in metal borohydrides and their ammoniates
Abstract
Ammoniation in metal borohydrides (MBs) with the form 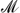 (BH4)x has been shown to lower their decomposition temperatures with
(BH4)x has been shown to lower their decomposition temperatures with 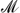 of low electronegativity (χp ≲ 1.6), but raise it for high-χp MBs (χp ≳ 1.6). Although this behavior is just as desired, an understanding of the mechanisms that cause it is still lacking. Using ab initio methods, we elucidate those mechanisms and find that ammoniation always causes thermodynamic destabilization, explaining the observed lower decomposition temperatures for low-χp MBs. For high-χp MBs, we find that ammoniation blocks B2H6 formation—the preferred decomposition mechanism in these MBs—and thus kinetically stabilizes those phases. The shift in decomposition pathway that causes the distinct change from destabilization to stabilization around χp = 1.6 thus coincides with the onset of B2H6 formation in MBs. Furthermore, with our analysis we are also able to explain why these materials release either H2 or NH3 gas upon decomposition. We find that NH3 is much more strongly coordinated with higher-χp metals and direct H2 formation/release becomes more favorable in these materials. Our findings are of importance for unraveling the hydrogen release mechanisms in an important new and promising class of hydrogen storage materials, allowing for a guided tuning of their chemistry to further improve their properties.
of low electronegativity (χp ≲ 1.6), but raise it for high-χp MBs (χp ≳ 1.6). Although this behavior is just as desired, an understanding of the mechanisms that cause it is still lacking. Using ab initio methods, we elucidate those mechanisms and find that ammoniation always causes thermodynamic destabilization, explaining the observed lower decomposition temperatures for low-χp MBs. For high-χp MBs, we find that ammoniation blocks B2H6 formation—the preferred decomposition mechanism in these MBs—and thus kinetically stabilizes those phases. The shift in decomposition pathway that causes the distinct change from destabilization to stabilization around χp = 1.6 thus coincides with the onset of B2H6 formation in MBs. Furthermore, with our analysis we are also able to explain why these materials release either H2 or NH3 gas upon decomposition. We find that NH3 is much more strongly coordinated with higher-χp metals and direct H2 formation/release becomes more favorable in these materials. Our findings are of importance for unraveling the hydrogen release mechanisms in an important new and promising class of hydrogen storage materials, allowing for a guided tuning of their chemistry to further improve their properties.



 Please wait while we load your content...
Please wait while we load your content...