Descriptor study by density functional theory analysis for the direct synthesis of hydrogen peroxide using palladium–gold and palladium–mercury alloy catalysts†
Abstract
Pd-based catalysts in which the Pd is alloyed with a transition metal (TM) are often considered for the direct synthesis of H2O2. In particular, PdAu and PdHg alloys are known for their good catalytic activities. However, finding suitable catalysts with designed compositions is not easy and the fundamental understanding of the mechanisms behind their enhanced activities is often lacking. In the current study, descriptor sets for direct H2O2 synthesis on Pd, PdAu, and PdHg surfaces were proposed based on density functional theory. By considering the surface electronic effects caused by the alloy surface composition, descriptor sets consisting of the adsorption energy for reaction intermediates such as O2, O, and OOH and activation energy barriers were derived from elementary reaction steps. The geometric factors of the adsorbed species were also considered, but found to be less important. The adsorption energies of O2 and O were calculated and compared to determine that surface-adsorbed 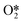 is the intermediate species required to form the desired product. The selectivity was assessed by comparing the adsorption energies of O and OOH. Considering the main thermodynamic and kinetic characteristics, the results showed that the PdHg alloy with a surface composition having the atomic ratio 2 : 1 gives the best selectivity. The results of the descriptor analysis indicated that alloying Pd with less active metals, such as Hg and Au, is a key strategy for designing catalysts with better catalytic activity and selectivity.
is the intermediate species required to form the desired product. The selectivity was assessed by comparing the adsorption energies of O and OOH. Considering the main thermodynamic and kinetic characteristics, the results showed that the PdHg alloy with a surface composition having the atomic ratio 2 : 1 gives the best selectivity. The results of the descriptor analysis indicated that alloying Pd with less active metals, such as Hg and Au, is a key strategy for designing catalysts with better catalytic activity and selectivity.



 Please wait while we load your content...
Please wait while we load your content...