Cooperative loading of multisite receptors with lanthanide containers: an approach for organized luminescent metallopolymers†
Abstract
Metal-containing (bio)organic polymers are materials of continuously increasing importance for applications in energy storage and conversion, drug delivery, shape-memory items, supported catalysts, organic conductors and smart photonic devices. The embodiment of luminescent components provides a revolution in lighting and signaling with the ever-increasing development of polymeric light-emitting devices. Despite the unique properties expected from the introduction of optically and magnetically active lanthanides into organic polymers, the deficient control of the metal loading currently limits their design to empirical and poorly reproducible materials. We show here that the synthetic efforts required for producing soluble multi-site host systems Lk are largely overcome by the virtue of reversible thermodynamics for mastering the metal loading with the help of only two parameters: (1) the affinity of the luminescent lanthanide container for a single binding site and (2) the cooperative effect which modulates the successive fixation of metallic units to adjacent sites. When unsymmetrical perfluorobenzene-trifluoroacetylacetonate co-ligands (pbta−) are selected for balancing the charge of the trivalent lanthanide cations, Ln3+, in six-coordinate [Ln(pbta)3] containers, the explored anti-cooperative complexation processes induce nearest-neighbor intermetallic interactions 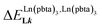 twice as large as thermal energy at room temperature (RT = 2.5 kJ mol−1). These values have no precedent when using standard symmetrical containers and they pave the way for programming metal alternation in luminescent lanthanidopolymers.
twice as large as thermal energy at room temperature (RT = 2.5 kJ mol−1). These values have no precedent when using standard symmetrical containers and they pave the way for programming metal alternation in luminescent lanthanidopolymers.



 Please wait while we load your content...
Please wait while we load your content...