How does chiral self-sorting take place in the formation of homochiral Pd6L8 capsules consisting of cyclotriveratrylene-based chiral tritopic ligands?†
Abstract
The chiral self-sorting process during the self-assembly of homochiral Pd6L8 capsules from cyclotriveratrylene (CTV)-based chiral tritopic ligands (L) and 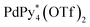 (Py*: 3-chloropyridine) was investigated by an NMR-based approach (QASAP: quantitative analysis of the self-assembly process). From the beginning to the formation of the
(Py*: 3-chloropyridine) was investigated by an NMR-based approach (QASAP: quantitative analysis of the self-assembly process). From the beginning to the formation of the 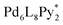 immature capsules (ICs), enantiomeric ligands are distributed in the intermediates in a non-self-sorting manner, which leads to the isomers of heterochiral ICs over 99% yield. The mismatch of the chirality in the heterochiral ICs prevents intramolecular ligand exchanges in ICs to form the heterochiral capsules. The correction of the chirality in the heterochiral ICs (chiral self-sorting) takes place very slowly to finally lead to the homochiral capsules. The reason why the chiral self-sorting took place in the late stage of the self-assembly (after the formation of the heterochiral ICs) would be due to the relatively high flexibility of the CTV-based ligand.
immature capsules (ICs), enantiomeric ligands are distributed in the intermediates in a non-self-sorting manner, which leads to the isomers of heterochiral ICs over 99% yield. The mismatch of the chirality in the heterochiral ICs prevents intramolecular ligand exchanges in ICs to form the heterochiral capsules. The correction of the chirality in the heterochiral ICs (chiral self-sorting) takes place very slowly to finally lead to the homochiral capsules. The reason why the chiral self-sorting took place in the late stage of the self-assembly (after the formation of the heterochiral ICs) would be due to the relatively high flexibility of the CTV-based ligand.



 Please wait while we load your content...
Please wait while we load your content...