Formation of defects and their effects on hydride ion transport properties in a series of K2NiF4-type oxyhydrides
Abstract
Several K2NiF4-type oxyhydrides (La2−x−ySrx+yLiH1−x+yO3−y) have in recent years been shown to exhibit significant transport of hydride ions. The migration mechanism of hydride ion conduction is, however, still not understood. In the following contribution, we explore the formation and stability of defects and the hydride ion migration mechanisms in three fast hydride ion conducting K2NiF4-type oxyhydrides (La2LiHO3, LaSrLiH2O2 and Sr2LiH3O) based on first principles calculations. Our results demonstrate that 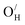 and
and 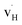 are the dominant defects, coexistent with minor amounts of
are the dominant defects, coexistent with minor amounts of 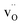 . Both
. Both 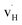 and
and 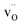 exhibit strong site preference at the axial sites of the LiX6 octahedron, showing that hydride ion transport is strongly anisotropic in the crystal lattice. Hydride ions migrate through a vacancy-mediated mechanism with calculated migration enthalpies varying from 0.21 to 0.45 eV depending on the composition of the oxyhydrides. By mapping all relevant minimum energy pathways for hydride and oxygen ions, we reveal that the transport of hydride ions in La2LiHO3 is inhibited by slow oxygen ion (vacancy) migration. For LaSrLiH2O2 and Sr2LiH3O,
exhibit strong site preference at the axial sites of the LiX6 octahedron, showing that hydride ion transport is strongly anisotropic in the crystal lattice. Hydride ions migrate through a vacancy-mediated mechanism with calculated migration enthalpies varying from 0.21 to 0.45 eV depending on the composition of the oxyhydrides. By mapping all relevant minimum energy pathways for hydride and oxygen ions, we reveal that the transport of hydride ions in La2LiHO3 is inhibited by slow oxygen ion (vacancy) migration. For LaSrLiH2O2 and Sr2LiH3O, 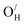 and
and 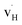 defect associates inhibit the hydride ion conduction further due to the strong binding energies of the complexes. However, the structural and compositional flexibility of K2NiF4-type oxyhydrides gives advantages for enhancing the hydride ion conductivity, for instance by introducing acceptor dopants on cation sites to create more vacant sites.
defect associates inhibit the hydride ion conduction further due to the strong binding energies of the complexes. However, the structural and compositional flexibility of K2NiF4-type oxyhydrides gives advantages for enhancing the hydride ion conductivity, for instance by introducing acceptor dopants on cation sites to create more vacant sites.



 Please wait while we load your content...
Please wait while we load your content...