Self-assembly pore-forming mechanism of foam boundary templates and the preparation of porous strontium hydroxyapatite microspheres by homogeneous precipitation
Abstract
Using ethylenediaminetetraacetic acid (EDTA) as a chelating agent and urea as a directional template, we report an effective method for the synthesis of porous strontium hydroxyapatite (SrHAp) microspheres employing homogeneous precipitation. More importantly, we can easily control the pore size (d) of porous SrHAp microspheres through the elaborate choice of reaction temperature (T) at atmospheric pressure. The experimental results show that the d of porous SrHAp microspheres rises gradually with the increase of T. In addition, with the increase of reaction time at the same T, the d of the microspheres remained unchanged, but the diameter of porous SrHAp microspheres gradually increased, and remained unchanged after 36 h. According to the Laplace equation, Kelvin equation and Clapeyron equation, the relationship of d and T is deduced as follows: 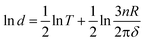 . The experimental data (T, d) agreed with it. In particular, based on the unique role of EDTA and CO2 bubbles (urea hydrolysis) in the preparation of porous SrHAp microspheres, the self-assembly pore-forming mechanism of foam boundary templates was proposed. First, urea was hydrolyzed to produce CO2 bubbles (foam boundary template) and OH−, and EDTASr (EDTA chelates strontium ions) and H3O+ (acidic system) formed a liquid film-chelating ion electronic double layer (LFCIEDL) on the surface of CO2 bubbles, which not only chelated and located Sr2+, but also formed a stable foam boundary template. Secondly, Sr2+, H2PO4− and OH− were homogeneously nucleated in the Plateau borders among the bubbles. After that, along the bubble liquid film surface, Sr2+ is orientationally released from the outer edge to the inner. SrHAp lamellae were formed by self-assembly method, then they were interconnected. In addition, OH− produced by urea hydrolysis continuously provides power for self-assembly oriented growth. Finally OH− completely destroys the LFCIEDL on the surface of the bubble, causing the bubble to break, and the porous SrHAp microspheres are formed.
. The experimental data (T, d) agreed with it. In particular, based on the unique role of EDTA and CO2 bubbles (urea hydrolysis) in the preparation of porous SrHAp microspheres, the self-assembly pore-forming mechanism of foam boundary templates was proposed. First, urea was hydrolyzed to produce CO2 bubbles (foam boundary template) and OH−, and EDTASr (EDTA chelates strontium ions) and H3O+ (acidic system) formed a liquid film-chelating ion electronic double layer (LFCIEDL) on the surface of CO2 bubbles, which not only chelated and located Sr2+, but also formed a stable foam boundary template. Secondly, Sr2+, H2PO4− and OH− were homogeneously nucleated in the Plateau borders among the bubbles. After that, along the bubble liquid film surface, Sr2+ is orientationally released from the outer edge to the inner. SrHAp lamellae were formed by self-assembly method, then they were interconnected. In addition, OH− produced by urea hydrolysis continuously provides power for self-assembly oriented growth. Finally OH− completely destroys the LFCIEDL on the surface of the bubble, causing the bubble to break, and the porous SrHAp microspheres are formed.



 Please wait while we load your content...
Please wait while we load your content...