Exploring chemical space with alchemical derivatives: alchemical transformations of H through Ar and their ions as a proof of concept†
Abstract
Alchemical derivatives have been used in recent years to obtain essentially qualitative information about transformations in which the number of electrons is unchanged. Within the context of Conceptual DFT, we present a systematic approach for combining changes in both the number of electrons 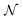 and the nuclear charge
and the nuclear charge 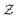 so that for example one can navigate from one neutral atom to another. A general formalism is presented for transformations involving changes both in
so that for example one can navigate from one neutral atom to another. A general formalism is presented for transformations involving changes both in 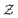 or
or 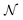 , where Parr's parabolic approach for the
, where Parr's parabolic approach for the 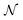 dependence is considered as one particular case and the ensemble description in the 0 K limit as the second case. The B3LYP functional in its CAMB3LYP version combined with the aug-cc-pCVQZ basis has been chosen to perform Coupled Perturbed Kohn Sham calculations of the alchemical derivatives. The monotonic behaviour of the alchemical potential is scrutinised. The order of magnitude analysis of the derivatives preludes convergence at third order. These results are injected in two strategies for obtaining transmutation energies from neutral atoms to a neighbouring neutral atom: one road moving along the
dependence is considered as one particular case and the ensemble description in the 0 K limit as the second case. The B3LYP functional in its CAMB3LYP version combined with the aug-cc-pCVQZ basis has been chosen to perform Coupled Perturbed Kohn Sham calculations of the alchemical derivatives. The monotonic behaviour of the alchemical potential is scrutinised. The order of magnitude analysis of the derivatives preludes convergence at third order. These results are injected in two strategies for obtaining transmutation energies from neutral atoms to a neighbouring neutral atom: one road moving along the 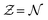 diagonal, the other one walking along a pure alchemical road after ionisation or electron attachment. Roads involving the anion of the reference atom are much less successful than those involving its cation. The transmutation energy for the cationic pathway displays chemical accuracy when the procedure is carried at third order in
diagonal, the other one walking along a pure alchemical road after ionisation or electron attachment. Roads involving the anion of the reference atom are much less successful than those involving its cation. The transmutation energy for the cationic pathway displays chemical accuracy when the procedure is carried at third order in 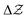 . The difficulties inherent to an accurate description of the anion and its response functions are responsible for the shortcomings along the anionic paths. As a direct application Ionization Energies (IE) and Electron Affinities (EA) are evaluated showing an almost perfect agreement with the direct evaluation and a difference with experimental values less than 0.5 eV for the IE. For the first EA reasonable agreement is obtained with direct and experimental values whereas the second EAs for atoms with stable mono-anions show a remarkable agreement with literature data. Besides proof of concept that with the information content of an atom one can get accurate energetics of its neighbours, the results indicate that alchemical derivatives are capable to yield quantitative information when navigating through Chemical Compound Space.
. The difficulties inherent to an accurate description of the anion and its response functions are responsible for the shortcomings along the anionic paths. As a direct application Ionization Energies (IE) and Electron Affinities (EA) are evaluated showing an almost perfect agreement with the direct evaluation and a difference with experimental values less than 0.5 eV for the IE. For the first EA reasonable agreement is obtained with direct and experimental values whereas the second EAs for atoms with stable mono-anions show a remarkable agreement with literature data. Besides proof of concept that with the information content of an atom one can get accurate energetics of its neighbours, the results indicate that alchemical derivatives are capable to yield quantitative information when navigating through Chemical Compound Space.

- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...