Thermodynamic targeting of electrocatalytic CO2 reduction: advantages, limitations, and insights for catalyst design†
Abstract
Herein is reported the electrocatalytic reduction of CO2 with the complex [Ni(bis-NHC)(dmpe)]2+ (1) (bis-NHC = 1,l′:3,3′-bis(1,3-propanediyl)dibenzimidazolin-2,2′-diylidene; dmpe = 1,2-bis(dimethylphosphino)ethane). The hydricity of 1 was previously benchmarked to be 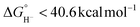 , equating to a driving force of a minimum of ∼3.4 kcal mol−1 for hydride transfer to CO2. While hydride transfer to CO2 is thermodynamically favorable, electrocatalytic and infrared spectroelectrochemical (IR-SEC) experiments reveal that hydride transfer is blocked by direct reactivity with CO2 in the reduced, Ni(0) state of the catalyst, yielding CO via reductive disproportionation (2CO2 + 2e− = CO + CO32−) and concomitant catalyst degradation. Although thermodynamic scaling relationships provide guidance in catalyst targeting, the findings herein illustrate the fundamental kinetic challenges in balancing substrate reactivity and selectivity in the design of CO2 reduction electrocatalysts. Advantages and limitations of this scaling relationship as well as approaches by which divergence from it may be achieved are discussed, which provides insight on important parameters for future catalyst design.
, equating to a driving force of a minimum of ∼3.4 kcal mol−1 for hydride transfer to CO2. While hydride transfer to CO2 is thermodynamically favorable, electrocatalytic and infrared spectroelectrochemical (IR-SEC) experiments reveal that hydride transfer is blocked by direct reactivity with CO2 in the reduced, Ni(0) state of the catalyst, yielding CO via reductive disproportionation (2CO2 + 2e− = CO + CO32−) and concomitant catalyst degradation. Although thermodynamic scaling relationships provide guidance in catalyst targeting, the findings herein illustrate the fundamental kinetic challenges in balancing substrate reactivity and selectivity in the design of CO2 reduction electrocatalysts. Advantages and limitations of this scaling relationship as well as approaches by which divergence from it may be achieved are discussed, which provides insight on important parameters for future catalyst design.



 Please wait while we load your content...
Please wait while we load your content...
