Partially fluorinated MIL-101(Cr): from a miniscule structure modification to a huge chemical environment transformation inspected by 129Xe NMR†
Abstract
The partial functionalisation of MIL-101(Cr) with fluorine was successfully achieved, for the first time, with 2,3,5,6-tetrafluoro-1,4-benzenedicarboxylate (BDC-4F) affording MIL-101(Cr)-4F(1%). As a result of the functionalisation the acidity of the metal centres for MIL-101(Cr)-4F(1%) was enhanced, which was measured by cyclic voltammetry. The resulting adsorption properties of the new material were explored and compared to those of MIL-101(Cr). H2O sorption experiments demonstrated an augmented hydrophobicity of the material due to the incorporation of fluorine atoms. CO2 adsorption experiments exhibited an augmented CO2 interaction energy (ΔH) for MIL-101(Cr)-4F(1%). The diffusion time constant 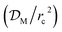 for CO2 showed a higher CO2 mobility inside the pores of MIL-101(Cr) than in MIL-101(Cr)-4F(1%), which was also associated with a higher selectivity (α) of MIL-101(Cr)-4F(1%) towards CO2. O2 sorption isotherms of MIL-101(Cr)-4F(1%), at a high pressure (up to 90 bar), demonstrated O2 uptakes among the highest values reported for a MOF material. H2 adsorption isotherms exhibited, at low pressures, higher H2 uptakes for the fluorine functionalised material. This was attributed to dipolar interactions of H2 molecules with the fluorine atoms and corroborated by the calculation of ΔH for H2. 129Xe NMR experiments were used to investigate the atypical pore-surface electron density of MIL-101(Cr)-4F(1%), demonstrating a huge effect of the polarising power of the fluorine atoms on the highly polarisable Xe atoms. Finally, the capture of I2 and H2S on MIL-101(Cr)-4F(1%) showed considerably higher values in comparison to MIL-101(Cr). This was attributed to the high dipolarity and polarisability of the fluorinated pores.
for CO2 showed a higher CO2 mobility inside the pores of MIL-101(Cr) than in MIL-101(Cr)-4F(1%), which was also associated with a higher selectivity (α) of MIL-101(Cr)-4F(1%) towards CO2. O2 sorption isotherms of MIL-101(Cr)-4F(1%), at a high pressure (up to 90 bar), demonstrated O2 uptakes among the highest values reported for a MOF material. H2 adsorption isotherms exhibited, at low pressures, higher H2 uptakes for the fluorine functionalised material. This was attributed to dipolar interactions of H2 molecules with the fluorine atoms and corroborated by the calculation of ΔH for H2. 129Xe NMR experiments were used to investigate the atypical pore-surface electron density of MIL-101(Cr)-4F(1%), demonstrating a huge effect of the polarising power of the fluorine atoms on the highly polarisable Xe atoms. Finally, the capture of I2 and H2S on MIL-101(Cr)-4F(1%) showed considerably higher values in comparison to MIL-101(Cr). This was attributed to the high dipolarity and polarisability of the fluorinated pores.



 Please wait while we load your content...
Please wait while we load your content...