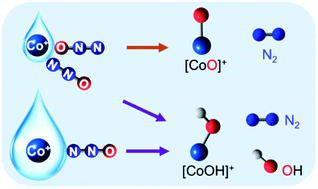
Hydrated cobalt(I) cluster ions, [Co(H2O)n]+, can decompose the inert nitrous oxide molecule, N2O. Density functional theory suggests that N2O can anchor to Co+ of [Co(N2O)(H2O)n]+ through either O end-on (η1-OL) or N end-on (η1-NL) coordinate mode. The latter is thermodynamically more favorable resulting from a subtle π backdonation from Co+ to N2O. N2O decomposition involves two major processes: (1) redox reaction and (2) N–O bond dissociation. The initial activation of N2O through an electron transfer from Co+ to N2O yields anionic N2O−, which binds to the metal center of [Co2+(N2O−)(H2O)n] also through either O end-on (η1-O) or N end-on (η1-N) mode and is stabilized by water molecules through hydrogen bonding. From η1-O, subsequent N–O bond dissociation to liberate N2, producing [CoO(H2O)n]+, is straightforward via a mechanism that is commonplace for typical metal-catalyzed N2O decompositions. Unexpectedly, the N–O bond dissociation directly from η1-N is also possible and eliminates both N2 and OH, explaining the formation of [CoOH(H2O)n]+ as observed in a previous experimental study. Interestingly, formation of [CoO(H2O)n]+ is kinetically controlled by the initial redox process between Co+ and the O-bound N2O, the activation barriers of which in large water clusters (n ≥ 14) are higher than that of the unexpected N–O bond dissociation from the N-bound structure forming [CoOH(H2O)n]+. This theoretical discovery implies that in the present of water molecules, the metal-catalyzed N2O decomposition starting from an O-bound metal complex is not mandatory.