Mechanistic understanding of the Cu(i)-catalyzed domino reaction constructing 1-aryl-1,2,3-triazole from electron-rich aryl bromide, alkyne, and sodium azide: a DFT study†
Abstract
The mechanism of the Cu(I)-catalyzed domino reaction furnishing 1-aryl-1,2,3-triazole assisted by CuI and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) is explored with density functional theory (DFT) calculations. The overall mechanism for this domino reaction comprises four consecutive reactions: (i) deprotonation of terminal alkyne by DBU; (ii) cycloaddition of copper acetylide and N3−; (iii) C–N bond coupling of the cuprate–triazole anion and aryl bromide; and (iv) protodecupration. Our theoretical calculations indicate that the Cu-catalyzed azidation of the electron-rich aryl bromides with N3−, which would generate the aryl azide for subsequent Cu(I)-catalyzed alkyne–azide cycloaddition, is not the dominant reaction because of its high free energy barrier. In addition, the cycloaddition process can assist C(aryl)–N bond formation. Activation strain analyses suggest that oxidative addition of aryl bromide onto the cuprate–triazole anion is more facile mainly due to enhanced dCu–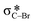 orbital interaction. A close attraction between copper and aryl bromide during oxidative addition is critical to the lower barrier.
orbital interaction. A close attraction between copper and aryl bromide during oxidative addition is critical to the lower barrier.



 Please wait while we load your content...
Please wait while we load your content...