Effect of temperature on the complexation of triscarbonatouranyl(vi) with calcium and magnesium in NaCl aqueous solution†
Abstract
The complex formation of triscarbonatouranyl(VI) UO2(CO3)34− with the alkaline earth metal ions Mg2+ and Ca2+ in 0.10 mol kgw−1 NaCl was studied at variable temperatures: 5–30 °C for Mg2+ and 10–50 °C for Ca2+. Under appropriate conditions, the ternary complexes (MnUO2(CO3)3(4−2n)− with n = 1 for Mg, n = {1; 2} for Ca) were identified by time-resolved laser-induced luminescence spectrometry. Their pure spectral components at 50 °C for CanUO2(CO3)3(4−2n)− and 30 °C for MgUO2(CO3)32− were recovered by multivariate curve resolution alternating least-squares analysis. Approximation models were tested to fit the experimental data—the equilibrium constants of complexation measured at different temperatures—and deduce the thermodynamic functions, i.e., enthalpy, entropy, and heat capacity. The weak influence of temperature on complexation constants induces large uncertainties in terms of thermodynamic functions. Assuming the enthalpy is constant with temperature using the Van't Hoff equation, the first stepwise complexation of UO2(CO3)34− by Ca2+ is estimated to be slightly endothermic, with 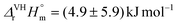 , while the second stepwise complexation of CaUO2(CO3)32− by Ca2+ with is slightly exothermic,
, while the second stepwise complexation of CaUO2(CO3)32− by Ca2+ with is slightly exothermic, 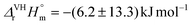 . In contrast to Ca2+, the complexation of UO2(CO3)34− by Mg2+ is slightly exothermic, with
. In contrast to Ca2+, the complexation of UO2(CO3)34− by Mg2+ is slightly exothermic, with 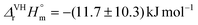 . These values are not significantly different from zero inasmuch as the uncertainties are important due to a weak dependence of log10 K° values. The entropic character of the complexation is verified as
. These values are not significantly different from zero inasmuch as the uncertainties are important due to a weak dependence of log10 K° values. The entropic character of the complexation is verified as 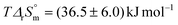 for the first stepwise complexation of UO2(CO3)34− by Ca2+,
for the first stepwise complexation of UO2(CO3)34− by Ca2+, 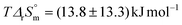 for the second stepwise complexation of CaUO2(CO3)32− by Ca2+, and
for the second stepwise complexation of CaUO2(CO3)32− by Ca2+, and 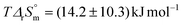 for the complexation of UO2(CO3)34− by Mg2+. The energetics of complexation and sensitivity analysis of the model estimates with temperature are discussed. The uranium speciation in the case of the safety of nuclear waste management, using the present thermodynamic functions, provides support to the assessment of underground nuclear waste repositories.
for the complexation of UO2(CO3)34− by Mg2+. The energetics of complexation and sensitivity analysis of the model estimates with temperature are discussed. The uranium speciation in the case of the safety of nuclear waste management, using the present thermodynamic functions, provides support to the assessment of underground nuclear waste repositories.



 Please wait while we load your content...
Please wait while we load your content...