Electrochemical studies of tris(cyclopentadienyl)thorium and uranium complexes in the +2, +3, and +4 oxidation states†
Abstract
Electrochemical measurements on tris(cyclopentadienyl)thorium and uranium compounds in the +2, +3, and +4 oxidation states are reported with C5H3(SiMe3)2, C5H4SiMe3, and C5Me4H ligands. The reduction potentials for both U and Th complexes trend with the electron donating abilities of the cyclopentadienyl ligand. Thorium complexes have more negative An(III)/An(II) reduction potentials than the uranium analogs. Electrochemical measurements of isolated Th(II) complexes indicated that the Th(III)/Th(II) couple was surprisingly similar to the Th(IV)/Th(III) couple in Cp′′-ligated complexes. This suggested that Th(II) complexes could be prepared from Th(IV) precursors and this was demonstrated synthetically by isolation of 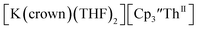 directly from
directly from 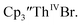 UV-visible spectroelectrochemical measurements and reactions of
UV-visible spectroelectrochemical measurements and reactions of 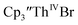 with elemental barium indicated that the thorium system undergoes sequential one electron transformations.
with elemental barium indicated that the thorium system undergoes sequential one electron transformations.



 Please wait while we load your content...
Please wait while we load your content...