A crossed molecular beams and computational study on the unusual reactivity of banana bonds of cyclopropane (c-C3H6;
 ) through insertion by ground state carbon atoms (C(3Pj))†
) through insertion by ground state carbon atoms (C(3Pj))†
Abstract
The mechanism and chemical dynamics of the reaction of ground electronic state atomic carbon C(3Pj) with cyclopropane c-C3H6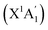 have been explored by combining crossed molecular beams experiments with electronic structure calculations of the pertinent triplet C4H6 potential energy surface and statistical computations of product branching ratios under single-collision conditions. The experimental findings suggest that the reaction proceeds via indirect scattering dynamics through triplet C4H6 reaction intermediate(s) leading to C4H5 product(s) plus atomic hydrogen via a tight exit transition state, with the overall reaction exoergicity evaluated as 231 ± 52 kJ mol−1. The calculations indicate that C(3Pj) can easily insert into one of the three equivalent C–C ‘banana’ bonds of cyclopropane overcoming a low barrier of only 2 kJ mol−1 following the formation of a van der Waals reactant complex stabilized by 15 kJ mol−1. The carbon atom insertion into one of the six C–H bonds is also feasible via a slightly higher barrier of 5 kJ mol−1. These results highlight an unusual reactivity of cyclopropane's banana C–C bonds, which behave more like unsaturated C–C bonds with a π-character than saturated σ C–C bonds, which are known to be generally unreactive toward the ground electronic state atomic carbon such as in ethane (C2H6). The statistical theory predicts the overall product branching ratios at the experimental collision energy as 50% for 1-butyn-4-yl, 33% for 1,3-butadien-2-yl, i-C4H5, and 11% for 1,3-butadien-1-yl, n-C4H5, with i-C4H5 (230 kJ mol−1 below the reactants) favored by the C–C insertion providing the best match with the experimentally observed reaction exoergicity. The C(3Pj) + c-C3H6 reaction is predicted to be a source of C4H5 radicals under the conditions where its low entrance barriers can be overcome, such as in planetary atmospheres or in circumstellar envelopes but not in cold molecular clouds. Both i- and n-C4H5 can further react with acetylene eventually producing the first aromatic ring and hence, the reaction of the atomic carbon with c-C3H6 can be considered as an initial step toward the formation of benzene.
have been explored by combining crossed molecular beams experiments with electronic structure calculations of the pertinent triplet C4H6 potential energy surface and statistical computations of product branching ratios under single-collision conditions. The experimental findings suggest that the reaction proceeds via indirect scattering dynamics through triplet C4H6 reaction intermediate(s) leading to C4H5 product(s) plus atomic hydrogen via a tight exit transition state, with the overall reaction exoergicity evaluated as 231 ± 52 kJ mol−1. The calculations indicate that C(3Pj) can easily insert into one of the three equivalent C–C ‘banana’ bonds of cyclopropane overcoming a low barrier of only 2 kJ mol−1 following the formation of a van der Waals reactant complex stabilized by 15 kJ mol−1. The carbon atom insertion into one of the six C–H bonds is also feasible via a slightly higher barrier of 5 kJ mol−1. These results highlight an unusual reactivity of cyclopropane's banana C–C bonds, which behave more like unsaturated C–C bonds with a π-character than saturated σ C–C bonds, which are known to be generally unreactive toward the ground electronic state atomic carbon such as in ethane (C2H6). The statistical theory predicts the overall product branching ratios at the experimental collision energy as 50% for 1-butyn-4-yl, 33% for 1,3-butadien-2-yl, i-C4H5, and 11% for 1,3-butadien-1-yl, n-C4H5, with i-C4H5 (230 kJ mol−1 below the reactants) favored by the C–C insertion providing the best match with the experimentally observed reaction exoergicity. The C(3Pj) + c-C3H6 reaction is predicted to be a source of C4H5 radicals under the conditions where its low entrance barriers can be overcome, such as in planetary atmospheres or in circumstellar envelopes but not in cold molecular clouds. Both i- and n-C4H5 can further react with acetylene eventually producing the first aromatic ring and hence, the reaction of the atomic carbon with c-C3H6 can be considered as an initial step toward the formation of benzene.

- This article is part of the themed collection: 2022 PCCP HOT Articles



 Please wait while we load your content...
Please wait while we load your content...

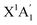 ) through insertion by ground state carbon atoms (C(3Pj))
) through insertion by ground state carbon atoms (C(3Pj))