Polyanionic cyano-fullerides for CO2 capture: a DFT prediction†
Abstract
The reaction of C60 fullerene with ‘n’ molecules (n = 1 to 6) of 1,3-dimethyl-2,3-dihydro-2-cyano-imidazole (IMCN) results in the exothermic formation of imidazolium cation-polyanionic fulleride complexes, (IM+)n⋯((C60(CN)n)n−). The binding energy of IM+ with (C60(CN)n)n− in the imidazolium-fulleride ionic complexes increased from −69.6 kcal mol−1 for n = 1 to −202.9 kcal mol−1 for n = 6. The energetics of the complex formation and cation–anion interaction energy data suggest the formation of imidazolium-fulleride ionic liquid (IL) systems. Furthermore, the dimer formation of such ionic complexes showed more exergonic nature due to multiple cooperative electrostatic interactions between oppositely charged species and suggested improved energetics for higher order clusters. The molecular electrostatic potential (MESP) analysis has revealed that the extra ‘n’ electrons in the ionic complex as well as that in the bare (C60(CN)n)n− are delocalized mainly on the unsaturated carbon centers of the fullerene unit, while the CN groups remain as a neutral unit. The MESP minimum (Vmin) values of (C60(CN)n)n− on the carbon cage have shown that the addition of each CN− unit on the cage enhances the negative character of Vmin by ∼54.7 kcal mol−1. This enhancement in MESP is comparable to the enhancement observed when one electron is added to C60 to produce 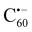 (−62.5 kcal mol−1) and suggests that adding ‘n’ CN− groups to the fullerene cage is equivalent to supplying ‘n’ electrons to the carbon cage. Also the high capacity of the fullerene cage to hold several electrons can be attributed to the spherical delocalization of them onto the electron deficient carbon cage. The interactive behavior of CO2 molecules with (IM+)n⋯(C60(CN)n)n− systems showed that the interaction becomes stronger from −2.3 kcal mol−1 for n = 1 to −18.6 kcal mol−1 for n = 6. From the trianionic fulleride onwards, the C⋯CO2 noncovalent (nc) interaction changes to C–CO2 covalent (c) interaction with the development of carboxylate character on the adsorbed CO2. These results prove that cyano-fullerides are promising candidates for CO2 capture.
(−62.5 kcal mol−1) and suggests that adding ‘n’ CN− groups to the fullerene cage is equivalent to supplying ‘n’ electrons to the carbon cage. Also the high capacity of the fullerene cage to hold several electrons can be attributed to the spherical delocalization of them onto the electron deficient carbon cage. The interactive behavior of CO2 molecules with (IM+)n⋯(C60(CN)n)n− systems showed that the interaction becomes stronger from −2.3 kcal mol−1 for n = 1 to −18.6 kcal mol−1 for n = 6. From the trianionic fulleride onwards, the C⋯CO2 noncovalent (nc) interaction changes to C–CO2 covalent (c) interaction with the development of carboxylate character on the adsorbed CO2. These results prove that cyano-fullerides are promising candidates for CO2 capture.



 Please wait while we load your content...
Please wait while we load your content...