The surface phase structure evolution of the fcc MoC (001) surface in a steam reforming atmosphere: systematic kinetic and thermodynamic investigations†
Abstract
The kinetic and thermodynamic aspects of the surface phase structure evolution of an fcc MoC (001) surface under a H2O/H2-rich atmosphere typically found during steam reforming processes were systematically studied via periodic density functional theory (DFT) and ab initio thermodynamic methods. The various stable configurations of surface species (H2O*, OH*, O*, 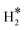 , and H*) at different coverages and their formation rates considering different coverage effects of certain species were explored. At a molecular H2O* adsorption coverage (θH2O) ≤1/3 ML, the adsorption of H2O mainly takes place through single Mo–O coordination, while the capture of H2O above 1/3 ML relies on hydrogen bonds. H2O* dissociation resulting in OH* formation is always facile at different H2O* coverages, whereas it becomes unfavourable as the OH* coverage increases beyond 4/9 ML due to unavoidable strong hydrogen bond breaking. Surface O* can be easily formed via hydroxyl disproportionation with negligible energy barriers, and the protonation of O* by H2O* is also facile. The dissociation of
, and H*) at different coverages and their formation rates considering different coverage effects of certain species were explored. At a molecular H2O* adsorption coverage (θH2O) ≤1/3 ML, the adsorption of H2O mainly takes place through single Mo–O coordination, while the capture of H2O above 1/3 ML relies on hydrogen bonds. H2O* dissociation resulting in OH* formation is always facile at different H2O* coverages, whereas it becomes unfavourable as the OH* coverage increases beyond 4/9 ML due to unavoidable strong hydrogen bond breaking. Surface O* can be easily formed via hydroxyl disproportionation with negligible energy barriers, and the protonation of O* by H2O* is also facile. The dissociation of 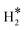 will easily generate surface Mo–H* and C–H* species, where Mo–H* can readily transform to C–H* with significant exothermicity. The average surface binding strengths of various species at 0 K follow the order: H2O* > H* ≈ OH* >
will easily generate surface Mo–H* and C–H* species, where Mo–H* can readily transform to C–H* with significant exothermicity. The average surface binding strengths of various species at 0 K follow the order: H2O* > H* ≈ OH* > 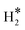 > O*, where the average binding strength of O* becomes positive when θO* ≥ 1/3 ML. At 473.15 K and over a wide H2O pressure range, mixtures of H2O*, OH*, and O* are the predominant species on the (001) surface, highlighting the role of the (001) surface in steam reforming reactions, while H* species only emerge at low H2O pressure or high H2 pressure. The proportion of O* species decreases and finally tends to zero as the H2 pressure increases from 10−10 to 10−7 MPa, while the proportion of OH* species increases due to O* protonation. As the H2 pressure increases from 10−7 to 10 MPa, the proportion of OH* species decreased, accompanied by an increase in the H2O coverage. As the H2O pressure decreased, the stable existence of surface H* species became increasingly more favorable, and the emergence of surface H* species was accompanied by the disappearance of surface O-containing species, changing the catalytic role of the (001) surface from catalyzing steam reforming processes to promoting hydrogenation reactions.
> O*, where the average binding strength of O* becomes positive when θO* ≥ 1/3 ML. At 473.15 K and over a wide H2O pressure range, mixtures of H2O*, OH*, and O* are the predominant species on the (001) surface, highlighting the role of the (001) surface in steam reforming reactions, while H* species only emerge at low H2O pressure or high H2 pressure. The proportion of O* species decreases and finally tends to zero as the H2 pressure increases from 10−10 to 10−7 MPa, while the proportion of OH* species increases due to O* protonation. As the H2 pressure increases from 10−7 to 10 MPa, the proportion of OH* species decreased, accompanied by an increase in the H2O coverage. As the H2O pressure decreased, the stable existence of surface H* species became increasingly more favorable, and the emergence of surface H* species was accompanied by the disappearance of surface O-containing species, changing the catalytic role of the (001) surface from catalyzing steam reforming processes to promoting hydrogenation reactions.



 Please wait while we load your content...
Please wait while we load your content...