Influence of the electronic effect of an ancillary ligand on MMCT and LMCT in localized cyanide-bridged complexes containing non-innocent ligands†
Abstract
Mixed-valence (MV) complexes containing non-innocent ligands are excellent models for the investigation of the electron-transfer process. A series of twelve bimetallic cyanide-bridged complexes [CpMen(dppe)RuCNFeLx][A] (A = PF6− or I−, CpMen = alkyl cyclopentadienyl, dppe = 1,2-bis (diphenylphosphino)ethane, and LX = pentane-2,4-dione-bis (S-alkylisothiosemi-carbazonato); n = 0, x = Methyl (Me), Ethyl (Et), n-Propyl (Pr) and n-Butyl (Bu), and A = PF6−, 1Me[PF6], 1Et[PF6], 1Pr[PF6], and 1Bu[PF6]; n = 1, x = Me, Et, Pr, and Bu, and A = PF6−, 2Me[PF6], 2Et[PF6], 2Pr[PF6], and 2Bu[PF6]; n = 5, x = Me, Et, Pr, and Bu, and A = I−, 3Me[I], 3Et[I], 3Pr[I], and 3Bu[I]) have been synthesized and well characterized. The investigations demonstrate that all the cations of the complexes could be described with the basic electronic configuration 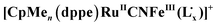 , in which the
, in which the 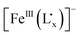 fragment could be regarded as being delocalized. The ligand to metal charge transfer (LMCT) transition in the
fragment could be regarded as being delocalized. The ligand to metal charge transfer (LMCT) transition in the 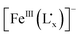 fragment and the low-spin RuII to the intermediate-spin FeIII charge transfer (MMCT) transition have been investigated. The UV-vis-NIR spectral analysis results suggest that the energy of the LMCT transition is lower than that of the MMCT transition due to electron delocalization between the non-innocent ligand
fragment and the low-spin RuII to the intermediate-spin FeIII charge transfer (MMCT) transition have been investigated. The UV-vis-NIR spectral analysis results suggest that the energy of the LMCT transition is lower than that of the MMCT transition due to electron delocalization between the non-innocent ligand 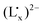 and the FeIII ion, which is strongly supported by TDDFT calculations. Furthermore, the RuII → FeIII MMCT energy decreases and the
and the FeIII ion, which is strongly supported by TDDFT calculations. Furthermore, the RuII → FeIII MMCT energy decreases and the 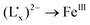 LMCT energy increases with the increasing electron donating ability of the ancillary ligands from Cp, CpMe to CpMe5, but slightly changes with the variation of the ligand Lx from Me, Et, Pr to Bu. Compared to the MMCT energy change, however, the energy of the LMCT from
LMCT energy increases with the increasing electron donating ability of the ancillary ligands from Cp, CpMe to CpMe5, but slightly changes with the variation of the ligand Lx from Me, Et, Pr to Bu. Compared to the MMCT energy change, however, the energy of the LMCT from 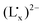 to FeIII in the delocalized
to FeIII in the delocalized 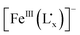 moiety is less influenced by the electronic effect of the ancillary ligand or the CpMen(dppe)RuIICN (n = 0, 1 and 5) fragment.
moiety is less influenced by the electronic effect of the ancillary ligand or the CpMen(dppe)RuIICN (n = 0, 1 and 5) fragment.



 Please wait while we load your content...
Please wait while we load your content...