Metalation-induced denitrogenative reductive coupling of isocyanides on a silylene-bridged nickel cluster†
Abstract
The denitrogenative reductive coupling of two molecules of CNtBu to afford a disilylketenimine with an aza-disilacyclobutane skeleton was achieved on a multinuclear silylene-bridged Ni cluster framework in the absence of any strong reducing reagents. During this reaction, sequential cleavage of a C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond and formation of a C
N bond and formation of a C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bond involving two molecules of CNtBu were achieved on a nickel cluster surrounded by four silylene moieties. First, the cleavage of the C
C bond involving two molecules of CNtBu were achieved on a nickel cluster surrounded by four silylene moieties. First, the cleavage of the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond of one molecule of CNtBu provided a silylene-supported carbide and an NtBu moiety on the dinuclear nickel skeleton. Further metalation induced coupling between the carbide moiety and an additional molecule of CNtBu on the pentanuclear nickel-cluster framework to form a
N bond of one molecule of CNtBu provided a silylene-supported carbide and an NtBu moiety on the dinuclear nickel skeleton. Further metalation induced coupling between the carbide moiety and an additional molecule of CNtBu on the pentanuclear nickel-cluster framework to form a 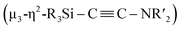 moiety via formation of a C
moiety via formation of a C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bond. Thermolysis of this pentanuclear cluster produced a disilylketenimine with an aza-disilacyclobutane skeleton in 58% yield.
C bond. Thermolysis of this pentanuclear cluster produced a disilylketenimine with an aza-disilacyclobutane skeleton in 58% yield.



 Please wait while we load your content...
Please wait while we load your content...