Polymorphism and phase transitions in Na2U2O7 from density functional perturbation theory
Abstract
Polymorphism and phase transitions in sodium diuranate, Na2U2O7, are investigated with density functional perturbation theory (DFPT). Thermal properties of crystalline α-, β- and γ-Na2U2O7 polymorphs are predicted from DFPT phonon calculations, i.e., the first time for the high-temperature γ-Na2U2O7 phase (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m symmetry). The standard molar isochoric heat capacities predicted within the quasi-harmonic approximation are
m symmetry). The standard molar isochoric heat capacities predicted within the quasi-harmonic approximation are 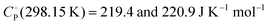 for P21/a α-Na2U2O7 and C2/m β-Na2U2O7, respectively. Gibbs free energy calculations reveal that α-Na2U2O7 (P21/a) and β-Na2U2O7 (C2/m) are almost energetically degenerate at low temperature, with β-Na2U2O7 becoming slightly more stable than α-Na2U2O7 as temperature increases. These findings are consistent with XRD data showing a mixture of α and β phases after cooling of γ-Na2U2O7 to room temperature and the observation of a sluggish α → β phase transition above ca. 600 K. A recently observed α-Na2U2O7 structure with P21 symmetry is also shown to be metastable at low temperature. Based on Gibbs free energy, no direct β → γ solid-solid phase transition is predicted at high temperature, although some experiments reported the existence of such phase transition around 1348 K. This, along with recent experiments, suggests the occurrence of a multi-step process consisting of initial β-phase decomposition, followed by recrystallization into γ-phase as temperature increases.
for P21/a α-Na2U2O7 and C2/m β-Na2U2O7, respectively. Gibbs free energy calculations reveal that α-Na2U2O7 (P21/a) and β-Na2U2O7 (C2/m) are almost energetically degenerate at low temperature, with β-Na2U2O7 becoming slightly more stable than α-Na2U2O7 as temperature increases. These findings are consistent with XRD data showing a mixture of α and β phases after cooling of γ-Na2U2O7 to room temperature and the observation of a sluggish α → β phase transition above ca. 600 K. A recently observed α-Na2U2O7 structure with P21 symmetry is also shown to be metastable at low temperature. Based on Gibbs free energy, no direct β → γ solid-solid phase transition is predicted at high temperature, although some experiments reported the existence of such phase transition around 1348 K. This, along with recent experiments, suggests the occurrence of a multi-step process consisting of initial β-phase decomposition, followed by recrystallization into γ-phase as temperature increases.



 Please wait while we load your content...
Please wait while we load your content...
