Frustrated amino functional group coupling with electric field makes CO2 activation easier†
Abstract
New models associated with frustrated geometry and an external electric field (EEF) were designed to qualitatively and quantitatively explore CO2 activation through density functional calculations. We investigated the influence on CO2 of the microenvironments of methylamine (CH3NH2) positioned at different heights above a Cu (111) surface in the presence and absence of an electric field. The results demonstrate that at an approximate distance of 4 ± 1 Å between N and the metal surface, neither lower nor higher, under an EEF over 0.4 V Å−1, there is a remarkable synergistic effect between the chemical interaction and EEF that activates CO2, and also lowers the required EEF strength. This is in contrast to the separate factors or any other combinations of them which do not achieve the synergistic effect. In addition, when H in 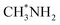 was replaced by F, the O–C–O angle of CO2 is not affected. This phenomenon further illustrates that the synergistic effect is very sensitive to the nucleophilicity of NH2. Various other chemical groups and substrates were investigated, and PHCH3 also displays a distinctive chemisorption CO2 state. The substrate also plays a significant role, except that Au cannot generate a similar effect. Furthermore, constraining or facilitating CO2 activation strongly depends on the distance between the chemical group and the substrate. Appropriate combinations of the three factors related to the substrate Cu, the chemical group CH3NH2 and the EEF provide new protocols to make CO2 activation easier and controllable.
was replaced by F, the O–C–O angle of CO2 is not affected. This phenomenon further illustrates that the synergistic effect is very sensitive to the nucleophilicity of NH2. Various other chemical groups and substrates were investigated, and PHCH3 also displays a distinctive chemisorption CO2 state. The substrate also plays a significant role, except that Au cannot generate a similar effect. Furthermore, constraining or facilitating CO2 activation strongly depends on the distance between the chemical group and the substrate. Appropriate combinations of the three factors related to the substrate Cu, the chemical group CH3NH2 and the EEF provide new protocols to make CO2 activation easier and controllable.



 Please wait while we load your content...
Please wait while we load your content...