Conditions to meet for the [CuOH]+ site to be favorable and reactive toward the conversion of methane to methanol over Cu-MOR zeolite†
Abstract
The nature of the active site in copper-exchanged zeolites catalyzing the direct conversion of CH4 to CH3OH has been a long yet important discussion. The mononuclear CuOH active site, in particular, has been a hot discussion, in addition to the mostly reported Cu2O and Cu3O3 active sites. While the binuclear and trinuclear species have been known to be stable and CH4 reactive, the mononuclear one remains unclear. Herein, we employ density functional theory (DFT) calculations to evaluate the stability and reactivity of CuOH-MOR zeolite toward CH4 oxidation to CH3OH. We find that the CuOH site is thermodynamically stable at low oxygen chemical potential and highly reactive with some conditions to meet: (i) the CuOH site must be hosted on specific Al sites and oriented parallel along the 12-membered ring of MOR to stabilize the resultant ˙CH3 radical and (ii) a Cu2+ inactive site must coexist adjacent to the CuOH active site to provide extra electron required to form CH3OH, although these two Cu monomers are prone to the formation of Cu–OH–Cu dimer when they are too close to each other. Furthermore, we also find that the stability of the 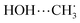 (in the homolytic C–H cleavage) or the C–Cu (in the heterolytic C–H cleavage) interaction formed during H–CH3 activation is mainly responsible for controlling the activation barrier. This paper clarifies previous contradictive results on the role, stability, and activity of the CuOH site and provides theoretical support and detailed elucidation for the recent experimental reports.
(in the homolytic C–H cleavage) or the C–Cu (in the heterolytic C–H cleavage) interaction formed during H–CH3 activation is mainly responsible for controlling the activation barrier. This paper clarifies previous contradictive results on the role, stability, and activity of the CuOH site and provides theoretical support and detailed elucidation for the recent experimental reports.
![Graphical abstract: Conditions to meet for the [CuOH]+ site to be favorable and reactive toward the conversion of methane to methanol over Cu-MOR zeolite](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D3CY00929G&imageInfo.ImageIdentifier.Year=2023)


 Please wait while we load your content...
Please wait while we load your content...