ANbO3 (A = Na, K) and
 (A′ = Ca, Sr) composite oxides for oxidative coupling of methane and oxidative dehydrogenation of ethane: perovskite vs. layered perovskite†
(A′ = Ca, Sr) composite oxides for oxidative coupling of methane and oxidative dehydrogenation of ethane: perovskite vs. layered perovskite†
Abstract
In this study, regular ANbO3 (A = Na, K) perovskites and layered 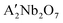 (A′ = Ca, Sr) perovskites have been successfully synthesized using the hydrothermal method for catalyzing oxidative coupling of methane (OCM) and oxidative dehydrogenation of ethane (ODHE). The findings reveal that unit cell free volume (Vf) is the most important factor for regular perovskites since it affects methane, ethane, and oxygen conversions. Meanwhile, the radii ratio of A and B atoms (rA/rB ratio) is the key parameter for layered perovskites and influences these conversions. Owing to the high-temperature phase transitions of regular perovskites, which generate oxygen vacancies and improve their oxygen mobility, the above-mentioned conversions are more profound in regular perovskites than those in layered perovskites. For OCM, chemisorbed oxygen species O2− and O22− are the selective oxygen species, while the surface lattice oxygen species are the completely oxidized hydrocarbon active species. For ODHE, surface O2− and chemisorbed oxygen species O2− are the selective oxygen species. During OCM, Vf and rA/rB ratio of regular and layered perovskites also affect C2 selectivity. This is because both these factors affect oxygen mobility, thereby influencing the generation of chemisorbed oxygen species and resulting in their C2 selectivity sequences being consistent with the methane, ethane, and oxygen conversions. During ODHE, the Nb–O bond strength is an important factor affecting C2H4 selectivity. Compared to regular perovskites, layered perovskites have longer and weaker Nb–O bonds. Meanwhile, the Nb–O bond is prone to fracture in both reactions, which results in more active lattice oxygen. Consequently, layered perovskites exhibit high COx selectivity during OCM, thus decreasing C2 selectivity. During ODHE, layered perovskites exhibit high C2H4 selectivity.
(A′ = Ca, Sr) perovskites have been successfully synthesized using the hydrothermal method for catalyzing oxidative coupling of methane (OCM) and oxidative dehydrogenation of ethane (ODHE). The findings reveal that unit cell free volume (Vf) is the most important factor for regular perovskites since it affects methane, ethane, and oxygen conversions. Meanwhile, the radii ratio of A and B atoms (rA/rB ratio) is the key parameter for layered perovskites and influences these conversions. Owing to the high-temperature phase transitions of regular perovskites, which generate oxygen vacancies and improve their oxygen mobility, the above-mentioned conversions are more profound in regular perovskites than those in layered perovskites. For OCM, chemisorbed oxygen species O2− and O22− are the selective oxygen species, while the surface lattice oxygen species are the completely oxidized hydrocarbon active species. For ODHE, surface O2− and chemisorbed oxygen species O2− are the selective oxygen species. During OCM, Vf and rA/rB ratio of regular and layered perovskites also affect C2 selectivity. This is because both these factors affect oxygen mobility, thereby influencing the generation of chemisorbed oxygen species and resulting in their C2 selectivity sequences being consistent with the methane, ethane, and oxygen conversions. During ODHE, the Nb–O bond strength is an important factor affecting C2H4 selectivity. Compared to regular perovskites, layered perovskites have longer and weaker Nb–O bonds. Meanwhile, the Nb–O bond is prone to fracture in both reactions, which results in more active lattice oxygen. Consequently, layered perovskites exhibit high COx selectivity during OCM, thus decreasing C2 selectivity. During ODHE, layered perovskites exhibit high C2H4 selectivity.




 Please wait while we load your content...
Please wait while we load your content...
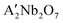 (A′ = Ca, Sr) composite oxides for oxidative coupling of methane and oxidative dehydrogenation of ethane: perovskite vs. layered perovskite
(A′ = Ca, Sr) composite oxides for oxidative coupling of methane and oxidative dehydrogenation of ethane: perovskite vs. layered perovskite