Stabilizing hydrogen-mediated sextuple bonds by quintuple superatomic bonding and a
 bond†
bond†
Abstract
Multiple bond orders of four and five are frequently obtained for d-block elements. However, compounds with sextuple bonding (2σ, 2π, and 2δ), for instance, Cr2, Mo2 and W2, are less stable and trapped only in the gas phase or inert matrices, probably resulting from large repulsion of two σ-type (σs and 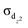 ) orbitals within the same zone. Herein, a superatomic bonding model is proposed to describe experimentally synthesized bridging hydride compounds. We theoretically predicted four unprecedented quintuple bridging hydride species with large EHL values, among which [(Cp)2Sc2(μ-H)5]− and [(Cp)2Mn2(μ-H)5]− (Cp = cyclopentadienyl) contain quintuple superatomic bonding (σ, 2π, and 2δ). In particular, two other species, [(Cp)2Ti2(μ-H)5]− and [(Cp)2V2(μ-H)5]+, were found to feature stable hydrogen-mediated sextuple bonding comprising a quintuple superatomic delocalized bond and an extra
) orbitals within the same zone. Herein, a superatomic bonding model is proposed to describe experimentally synthesized bridging hydride compounds. We theoretically predicted four unprecedented quintuple bridging hydride species with large EHL values, among which [(Cp)2Sc2(μ-H)5]− and [(Cp)2Mn2(μ-H)5]− (Cp = cyclopentadienyl) contain quintuple superatomic bonding (σ, 2π, and 2δ). In particular, two other species, [(Cp)2Ti2(μ-H)5]− and [(Cp)2V2(μ-H)5]+, were found to feature stable hydrogen-mediated sextuple bonding comprising a quintuple superatomic delocalized bond and an extra 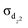 localized bond. Thereinto, the superatomic σs bond disperses to the outer space for better overlap with the orbitals of bridging H atoms, thus leaving the inner regions with low electron densities and providing adequate space for the
localized bond. Thereinto, the superatomic σs bond disperses to the outer space for better overlap with the orbitals of bridging H atoms, thus leaving the inner regions with low electron densities and providing adequate space for the 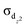 bond. These two quintuple bridging hydride compounds are verified to have thermal stability by molecular dynamics simulations and expected to provide an effective strategy to synthesize molecules with stable hydrogen-mediated sextuple bonding under normal experimental conditions.
bond. These two quintuple bridging hydride compounds are verified to have thermal stability by molecular dynamics simulations and expected to provide an effective strategy to synthesize molecules with stable hydrogen-mediated sextuple bonding under normal experimental conditions.




 Please wait while we load your content...
Please wait while we load your content...
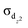 bond
bond