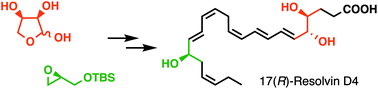
The total synthesis of Resolvin D4 and its 17(R)-hydroxy-epimer is reported. These lipid-based natural products are biosynthesized from docosahexaenoic acid (DHA, C22:6) during the body's rapid cellular and chemical response to injurious stimuli and are part of a large class of bioactive molecules that resolve inflammation. Our convergent synthesis employed a chiral pool strategy starting from glycidol derivatives and D-erythrose to introduce stereogenic centers. A copper(I)-mediated cross coupling between propargyl bromide and terminal acetylenic precursors yielded core structures of late-stage key intermediates. A simultaneous Lindlar reduction of the skipped diynyl moiety followed by silyl group cleavage securely completed the synthesis. The synthetic availability of these molecules helped further elucidate their stereoselective biofunctions.