Chlorination of trichlorosilane/chlorodimethylsilane using metal chlorides: experimental and mechanistic investigations
Abstract
Removal of carbonaceous impurities from trichlorosilane (SiHCl3) reduces the carbon content of solar grade polysilicon produced with the improved Siemens method. The separation of chlorodimethylsilane (CH3)2SiHCl from SiHCl3 by distillation remains challenging due to the small difference in their boiling points. Herein, the chlorination of (CH3)2SiHCl/SiHCl3 with metal chlorides (WCl6, MoCl5) were studied. The aim was to convert (CH3)2SiHCl into (CH3)2SiCl2, increase the relative volatility of (CH3)2SiHCl and SiHCl3 and facilitate the distillation. The optimum reaction conditions were 60 °C, 60 min and n(WCl6 or MoCl5): n(SiHCl3 or (CH3)2SiHCl) = 0.7 at 0.8 MPa. Under these conditions, and when WCl6 and MoCl5 were used as the chlorine sources, the extents of (CH3)2SiHCl conversion were 22.7 and 18.5 times higher than those of SiHCl3, respectively. In addition, a mechanistic study showed that the difference between the reactions of SiHCl3 and (CH3)2SiHCl resulted from the different energy barriers for the reactions of the 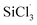 and (CH3)2SiCl· radicals with WClx or MoClx, and the barrier for the
and (CH3)2SiCl· radicals with WClx or MoClx, and the barrier for the 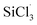 reaction was higher than that for the (CH3)2SiCl· reaction.
reaction was higher than that for the (CH3)2SiCl· reaction.



 Please wait while we load your content...
Please wait while we load your content...