Hypervalent iodine-promoted twofold oxidative coupling of amines with amides and thioamides: chemoselective pathway to oxazoles and thiazoles†
Abstract
Direct functionalization of the C(O)–N amide bond is one of the most high-profile research directions in the last few decades; however oxidative couplings involving amide bonds and functionalization of thioamide C(S)–N analogues remain an unsolved challenge. Herein, a novel hypervalent iodine-induced twofold oxidative coupling of amines with amides and thioamides has been established. The protocol accomplishes divergent C(O)–N and C(S)–N disconnection by the previously unknown Ar–O and Ar–S oxidative coupling and highly chemoselectively assembles the versatile yet synthetically challenging oxazoles and thiazoles. Employing amides instead of thioamides affords an alternative bond cleavage pattern, which is a result of the higher 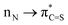 conjugation in thioamides. Mechanistic investigations indicate ureas and thioureas generated in the first oxidation as pivotal intermediates to realize the oxidative coupling. These findings open up new avenues for exploring oxidative amide and thioamide bond chemistry in various synthetic contexts.
conjugation in thioamides. Mechanistic investigations indicate ureas and thioureas generated in the first oxidation as pivotal intermediates to realize the oxidative coupling. These findings open up new avenues for exploring oxidative amide and thioamide bond chemistry in various synthetic contexts.



 Please wait while we load your content...
Please wait while we load your content...