Gas-phase preparation of azulene (C10H8) and naphthalene (C10H8) via the reaction of the resonantly stabilized fulvenallenyl (C7H5˙) and propargyl (C3H3˙) radicals†
Abstract
Synthetic routes to the 10π Hückel aromatic azulene (C10H8) molecule, the simplest polycyclic aromatic hydrocarbon carrying an adjacent five- and seven-membered ring, have been of fundamental importance due to the role of azulene – a structural isomer of naphthalene – as an essential molecular building block of saddle-shaped carbonaceous nanostructures such as curved nanographenes and nanoribbons. Here, we report on the very first gas phase preparation of azulene by probing the gas-phase reaction between two resonantly stabilized radicals, fulvenallenyl 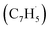 and propargyl
and propargyl 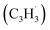 , in a molecular beam through isomer-resolved vacuum ultraviolet photoionization mass spectrometry. Augmented by electronic structure calculations, the novel Fulvenallenyl Addition Cyclization Aromatization (FACA) reaction mechanism affords a versatile concept for introducing the azulene moiety into polycyclic aromatic systems thus facilitating an understanding of barrierless molecular mass growth processes of saddle-shaped aromatics and eventually carbonaceous nanoparticles (soot, interstellar grains) in our universe.
, in a molecular beam through isomer-resolved vacuum ultraviolet photoionization mass spectrometry. Augmented by electronic structure calculations, the novel Fulvenallenyl Addition Cyclization Aromatization (FACA) reaction mechanism affords a versatile concept for introducing the azulene moiety into polycyclic aromatic systems thus facilitating an understanding of barrierless molecular mass growth processes of saddle-shaped aromatics and eventually carbonaceous nanoparticles (soot, interstellar grains) in our universe.



 Please wait while we load your content...
Please wait while we load your content...