Effects of state filling and localization on chemical expansion in praseodymium-oxide perovskites†
Abstract
In oxide materials, an increase in oxygen vacancy concentration often results in lattice expansion, a phenomenon known as chemical expansion that can introduce detrimental stresses and lead to potential device failure. One factor often implicated in the chemical expansion of materials is the degree of localization of the multivalent cation electronic states. When an oxygen 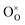 is removed from the lattice and a vacancy
is removed from the lattice and a vacancy 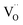 forms, it is believed that the two released electrons reduce multivalent cations and expand the lattice, with more localized cation states resulting in larger expansion. In this work, we computationally and experimentally studied the chemical expansion of two Pr-based perovskites that exhibit ultra-low chemical expansion, PrGa1−xMgxO3−δ and BaPr1−xYxO3−δ, and their parent compounds PrGaO3−δ and BaPrO3−δ. Using density functional theory, the degree of localization of the Pr-4f electrons was varied by adjusting the Hubbard U parameter. We find that the relationship between Pr-4f electron localization and chemical expansion exhibits more complexity than previously established. This relationship depends on the nature of the states filled by the two electrons, which may not necessarily involve the reduction of Pr. F′-center defects can form if the reduction of Pr is unfavorable, leading to smaller chemical expansions. If hole states are present in the material, the states filled by the electrons can be Pr-4f and/or O-2p hole states depending on the degree of Pr-4f localization. The O-2p holes are more delocalized than the Pr-4f holes, resulting in smaller chemical expansions when the O-2p holes are filled. X-ray photoelectron spectroscopy reveals low concentrations of Pr4+ in PrGa0.9Mg0.1O3−δ and BaPr0.9Y0.1O3−δ, supporting the possible role of O-2p holes in the low chemical expansions exhibited by these materials. This work highlights the non-trivial effects of electron localization on chemical expansion, particularly when hole states are present, pointing to design strategies to tune the chemical expansion of materials.
forms, it is believed that the two released electrons reduce multivalent cations and expand the lattice, with more localized cation states resulting in larger expansion. In this work, we computationally and experimentally studied the chemical expansion of two Pr-based perovskites that exhibit ultra-low chemical expansion, PrGa1−xMgxO3−δ and BaPr1−xYxO3−δ, and their parent compounds PrGaO3−δ and BaPrO3−δ. Using density functional theory, the degree of localization of the Pr-4f electrons was varied by adjusting the Hubbard U parameter. We find that the relationship between Pr-4f electron localization and chemical expansion exhibits more complexity than previously established. This relationship depends on the nature of the states filled by the two electrons, which may not necessarily involve the reduction of Pr. F′-center defects can form if the reduction of Pr is unfavorable, leading to smaller chemical expansions. If hole states are present in the material, the states filled by the electrons can be Pr-4f and/or O-2p hole states depending on the degree of Pr-4f localization. The O-2p holes are more delocalized than the Pr-4f holes, resulting in smaller chemical expansions when the O-2p holes are filled. X-ray photoelectron spectroscopy reveals low concentrations of Pr4+ in PrGa0.9Mg0.1O3−δ and BaPr0.9Y0.1O3−δ, supporting the possible role of O-2p holes in the low chemical expansions exhibited by these materials. This work highlights the non-trivial effects of electron localization on chemical expansion, particularly when hole states are present, pointing to design strategies to tune the chemical expansion of materials.

- This article is part of the themed collection: 2023 Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...
