Dopant-induced changes of local structures for adjusting the hydration ability of proton-conducting lanthanum scandates†
Abstract
Despite the well-known increase in basicity in the CaO–SrO–BaO simple oxide series, acceptor doping in La0.95M0.05ScO3−δ (M = Ca, Sr, Ba) orthorhombically distorted perovskites demonstrates the opposite trend. In the Ca–Sr–Ba dopant series, the standard enthalpy of the hydration reaction decreases and the mobility of protons increases. At first glance, a decrease in the electronegativity of a dopant should lead to improved hydration. However, we show that the dopant-induced local distortions in the crystal structure and the electronic structure of the material influence the hydration the most. The greater depth of local levels in the band gap for polarons bound with an oxygen vacancy or an acceptor dopant reflects the greater energy of 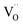 and OH· defect formation, while the shortest O–O interatomic distances determine better proton site stability, which jointly affects the hydration ability of the material. The combination of high proton stability and mobility leads to Sr as the optimal dopant for the operating conditions of proton-ceramic fuel cells (773–973 K).
and OH· defect formation, while the shortest O–O interatomic distances determine better proton site stability, which jointly affects the hydration ability of the material. The combination of high proton stability and mobility leads to Sr as the optimal dopant for the operating conditions of proton-ceramic fuel cells (773–973 K).



 Please wait while we load your content...
Please wait while we load your content...