The mechanism of CO2 hydrogenation to CH3OH on MZrOx (M = Ga, Cr) solid-solution catalysts and effects of lattice strain†
Abstract
CO2 hydrogenation to methanol is an effective way to convert CO2 into useful chemicals and fuel. Recently, some of metal-doped zirconia solid-solutions exhibit high selectivity and activity for CO2 hydrogenation to methanol. Herein, we report a mechanistic study of the process on MZrOx (M = Ga, Cr) using density functional calculations and microkinetic simulations. It is found that the pathway for methanol formation is 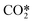 → COOH* → HCOOH* → CHO* → CH2O* → CH2OH* → CH3OH* and HCOOH* → CHO* + OH* is the rate-controlling step on both catalysts. The calculated methanol selectivity agrees with the experimental results very well, supporting the mechanism identified. Investigations show that the influence of strain on reaction kinetics and thermodynamics is not “black or white”: while compressive strain greatly promotes the activity and selectivity of GaZrOx, both tensile and compressive strains suppress the catalytic performance of CrZrOx. Analysis also reveals that there is a good transition state scaling (TSS) relation and strain tends to degrade the linear correlation. Furthermore, the error of the predicted barriers using the TSS relation is relatively large, indicating that cautions should be taken when applying TSS relations to estimate barriers.
→ COOH* → HCOOH* → CHO* → CH2O* → CH2OH* → CH3OH* and HCOOH* → CHO* + OH* is the rate-controlling step on both catalysts. The calculated methanol selectivity agrees with the experimental results very well, supporting the mechanism identified. Investigations show that the influence of strain on reaction kinetics and thermodynamics is not “black or white”: while compressive strain greatly promotes the activity and selectivity of GaZrOx, both tensile and compressive strains suppress the catalytic performance of CrZrOx. Analysis also reveals that there is a good transition state scaling (TSS) relation and strain tends to degrade the linear correlation. Furthermore, the error of the predicted barriers using the TSS relation is relatively large, indicating that cautions should be taken when applying TSS relations to estimate barriers.



 Please wait while we load your content...
Please wait while we load your content...